
Pathological response of ovarian cancer to neoadjuvant chemotherapy
Use of neoadjuvant therapy for cancer treatment may have several purposes. First, it is aimed to reduce tumor burden and thus to permit less extensive surgical intervention. Secondly, neoadjuvant therapy is believed to be an in vivo test for evaluation of tumor sensitivity to a given drug scheme, which helps to adjust further treatment options. Thirdly, neoadjuvant therapy can be utilized for the trials of novel anticancer agents as it deals with chemonaive malignancies and provides an opportunity to investigate surgically excised drug-exposed tumor material.
Most of concepts devoted to the use of neoadjuvant chemotherapy (NACT) were developed in the framework of breast cancer (BC) studies (1,2). One of the goals of applying NACT for BC treatment is to save the cosmetic appearance of breast by utilizing organ-preserving surgery. While applying for BC, particularly its triple-negative subtype, NACT relatively often results in pathologic complete response (pCR), which is associated with low probability of tumor relapse and justifies abstinence from adjuvant therapy. Furthermore, the results of BC NACT trials are considered by regulatory bodies for the registration of novel cytotoxic and targeted agents (3).
The application of NACT for the treatment of ovarian cancer (OC) is different from the one in BC in many respects. Overall, there is limited diversity with regard to treatment schemes: use of the doublet consisting of carboplatin and paclitaxel is currently the only widely accepted scheme, with little or no variation observed between different clinics (Table 1). NACT remains the treatment of choice mainly for those patients, who technically cannot be subjected to complete primary surgical debulking, either due to extensive disease spread or because of high risk of serious perioperative complications (18). pCR of OC to NACT are very rare; not surprisingly, unlike in case of BC, most of OC patients relapse even after complete surgical debulking (Table 1).

Full table
The response to primary therapy largely depends on OC histological type. High-grade serous OC (HGSOC) are characterized by a relatively high sensitivity to carboplatin-paclitaxel therapy, while other OC entities have limited responsiveness to this regimen. Earlier NACT studies involved OC patients of different histological types, with HGSOC constituting the majority but not all OC cases included (4,5,8,10). Recent investigations focus on the analysis HGSOC, which allows more balanced interstudy comparison (12-16).
There is a limited number of studies devoted to the analysis of OC pathologic response to NACT. Some of these reports attempted to evaluate the rates of pCR; while ranging from 0% to 4–14%, pCR demonstrated clear association with improved outcome (6-8,10,11,17). Recently accepted Chemotherapy Response Scoring (CRS, see below) system pools together complete and near-complete pathological responses (12). Although it is advised by the inventors of CRS to mention instances of complete pathological responses, subsequent confirmatory CRS studies did not specifically address this issue. Another difficulty in the assessing the true rate of pCR lies in the extent of the pathological analysis. OC surgery usually involves high amount of excised material; by definition, as more sites are subjected to morphological evaluation, as there are more chances to find small residual tumor cell clusters even in cases with exhaustive gross response. It is difficult to specify minimal extent of the required pathological analysis, but it is self-explanatory that the diagnosis of pCR has to be based on a very thorough examination of all resected tissues. Given the rarity of pCR in OC after NACT, there are no appropriate clinical studies assessing further treatment options for these patients. In theory, abstinence from consequent adjuvant therapy can be considered for women with reliably established pCR; indeed, OC has little propensity to distant metastases, therefore lack of viable tumor cells in the sites of surgery may be considered as a surrogate for cure. It is necessary to keep in mind, that the currently used microscopic analysis of surgical material has some sensitivity limit. It remains to be established, whether instances of morphologically detected pCR will hold true upon the use of modern mutation-based methods for detection of single tumor cells.
While the majority of studies assessing pCR in HGSOC after NACT involved unselected patients exposed to paclitaxel and carboplatin doublet, there is a clinical study comparing the efficacy of distinct NACT schemes in BRCA1-driven OC patients (17). Although cancers arising in BRCA1 germ-line mutation carriers are characterized by increased sensitivity of the gross tumor mass to conventional chemotherapy (19), no pCR has been recorded in women receiving carboplatin plus paclitaxel, cisplatin plus cyclophosphamide, cisplatin plus cyclophosphamide plus doxorubicin or single-agent cisplatin. However, 2/12 (17%) BRCA1-mutated OC demonstrated pCR to the combination of cisplatin and mitomycin C (17).
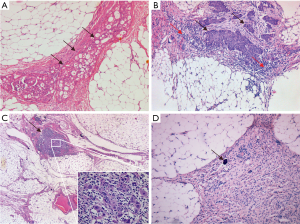
The analysis of non-pCR responses to NACT involves relatively complicated grading systems. Overall, they consider the size and visual appearance of tumor foci as well as the degree of response-associated inflammatory, fibrotic and necrotic changes. Some studies suggested that evident post-NACT morphological changes are associated with improved disease outcomes (4,5,9,10), while Ferron et al. (8) and Petrillo et al. (11) stated that only pCR is of real prognostic value. Some standardization of the assessment of pathological response score is attributed to the recent work of Böhm et al. (12) who suggested a 3-tier score assessing the status of omental tumor dissemination (Figure 1). CRS1 (no or minimal tumor response) describes post-NACT carcinomas with high prevalence of viable tumor cells with no or minimal regression-associated fibroinflammatory changes. CRS2 (appreciable tumor response amid viable tumor) corresponds to situations, where viable tumor foci can be readily identified among regression-associated fibroinflammatory changes. CRS3 (complete or near-complete response) is manifested either by the lack of residual tumor cells or presence of tumor foci up to 2 mm maximum size. According to the report of Böhm et al. (12) and some confirmatory studies, omental CRS3 is observed in approximately 30–40% HGSOC patients treated by NACT. Omental CRS3 is reproducibly associated with the improvement of the disease prognosis, while the differences in outcomes between women with CRS1 and CRS2 are minimal (12-16). Chemotherapy Response Scoring is included in the recommendations of the International Collaboration on Cancer Reporting (20) and there is an internet site facilitating appropriate training of pathologists (21).
Although the assessment of pathologic response after NACT is intuitively appealing, there are no clear guidelines which suggest clinical actions towards poor responders. Therefore, it is difficult to articulate, how the post-NACT morphological analysis affects the management of a given patient. Interestingly, recent study offered additional cytotoxic agent (capecitabine) to BC patients, who failed to achieve pCR upon NACT; this attempt to cope with NACT-resistant residual tumor clones resulted in improved disease outcomes (22). In contrast to this experimental approach, the composition of NACT and adjuvant therapy in OC patients is usually identical, irrespective of the degree of pathologic response. It is recommended to evaluate the clinical utility of OC pathologic response assessment in prospective randomized NACT trials (23).
The treatment of advanced OC was long considered to be a more or less linear process, where NACT is provided in order to convert non-resectable disease into an operable condition, surgery is aimed to further reduce the gross tumor bulk, and, in ideal situation, to achieve complete tumor resection, and the adjuvant therapy is administered for the sake of elimination of residual invisible tumor cells. Given that the majority of HGSOC rapidly shrink in response to first cycles of NACT, one would expect that this strategy is likely to result in the cure of these highly chemosensitive tumors. In contrast to these assumptions, very most HGSOC threated by NACT, surgery and adjuvant therapy relapse within a year after the completion of adjuvant therapy. Recent study of Sokolenko et al. (24) provides an explanation for these counterintuitive outcomes.
Sokolenko et al. (24) analysed a group of BRCA1-mutated HGSOC, which were treated by platinum-based NACT. BRCA1-driven tumors are characterized by the somatic loss of the remaining BRCA1 allele, which results in tumor-selective BRCA1 deficiency and is accompanied by pronounced vulnerability of cancer cells to platinum drugs (19). As expected, the majority (17/23, 74%). of treatment-naïve HGSOC exhibited loss-of-heterozygosity (LOH) in BRCA1 locus. However, when the authors analysed tumor specimens obtained after NACT, 11 (65%) out of 17 initially LOH-positive HGSOC demonstrated the restoration of BRCA1 heterozygosity. The subsequent molecular analysis reliably revealed that the gain of BRCA1 function in the residual tumor is attributed not to the second mutation in BRCA1 gene, but to the rapid selection of pre-existing BRCA1 proficient cells. This observation has a number of far-reaching outcomes, especially if we consider that even sporadic HGSOC often develop via somatic loss of BRCA1 function.
First of all, it demonstrates that long-term management of cancer disease is not a linear process. In fact, it may take just a few weeks for an initially chemosensitive tumor mass to become repopulated by drug-resistant tumor clones. If post-NACT BRCA1-driven tumors no longer have a molecular target for platinum therapy, it is hard to expect that the continuation of the same therapy in the adjuvant setting will reduce the risk of relapse. Furthermore, the described findings call to reconsider the mission of pathological response assessment. If we believe, that the tumor shrinkage occurs in a more or less continuous way, the evaluation of tumor morphology at the end of NACT will indeed provide a valuable snapshot of the response to the therapy. However, if we assume that the post-NACT cancer mass consists of highly selected population of malignant cells, who succeeded to adapt to continuous exposure of cytotoxic drugs, their mere presence in the surgically removed tissues is likely to be a fatal sign, irrespectively of the visual appearance of tumor clones. The latter supposition is well compatible with the data of Sokolenko et al. (24), who observed perfectly viable cancer cells in post-NACT tissues despite excellent gross response to the therapy.
Overall, the fact of low pCR rate in OC is a clear indicator of limited long-term efficacy of existing systemic treatment options for advanced OC. Early-stage clinical trials involving novel drugs are usually performed on heavily pretreated OC patients, who are lacking standard therapeutic opportunities. Pathological and molecular findings demonstrate, that the tumor mass may critically change its biological properties during the treatment, due to selective pressure of anticancer therapy. Therefore, data obtained on heavily pretreated cancer patients cannot be extrapolated to the earlier lines of therapeutic intervention. Neoadjuvant drug trials, aimed at the increase of pCR rates, may facilitate the evaluation of novel drug schemes for OC treatment.
Acknowledgements
Funding: This work is supported by the Russian Science Foundation (grant 17-75-30027).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Steenbruggen TG, van Ramshorst MS, Kok M, et al. Neoadjuvant Therapy for Breast Cancer: Established Concepts and Emerging Strategies. Drugs 2017;77:1313-36. [Crossref] [PubMed]
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry. Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. Available online: [accessed 20 July 2018].https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM305501.pdf
- Zardavas D, Piccart M. Neoadjuvant therapy for breast cancer. Annu Rev Med 2015;66:31-48. [Crossref] [PubMed]
- Le T, Williams K, Senterman M, et al. Histopathologic assessment of chemotherapy effects in epithelial ovarian cancer patients treated with neoadjuvant chemotherapy and delayed primary surgical debulking. Gynecol Oncol 2007;106:160-3. [Crossref] [PubMed]
- Le T, Williams K, Senterman M, et al. Omental chemotherapy effects as a prognostic factor in ovarian cancer patients treated with neoadjuvant chemotherapy and delayed primary surgical debulking. Ann Surg Oncol 2007;14:2649-53. [Crossref] [PubMed]
- Sassen S, Schmalfeldt B, Avril N, et al. Histopathologic assessment of tumor regression after neoadjuvant chemotherapy in advanced-stage ovarian cancer. Hum Pathol 2007;38:926-34. [Crossref] [PubMed]
- Miller K, Price JH, Dobbs SP, et al. An immunohistochemical and morphological analysis of post-chemotherapy ovarian carcinoma. J Clin Pathol 2008;61:652-7. [Crossref] [PubMed]
- Ferron JG, Uzan C, Rey A, et al. Histological response is not a prognostic factor after neoadjuvant chemotherapy in advanced-stage ovarian cancer with no residual disease. Eur J Obstet Gynecol Reprod Biol 2009;147:101-5. [Crossref] [PubMed]
- Samrao D, Wang D, Ough F, et al. Histologic parameters predictive of disease outcome in women with advanced stage ovarian carcinoma treated with neoadjuvant chemotherapy. Transl Oncol 2012;5:469-74. [Crossref] [PubMed]
- Muraji M, Sudo T, Iwasaki S, et al. Histopathology predicts clinical outcome in advanced epithelial ovarian cancer patients treated with neoadjuvant chemotherapy and debulking surgery. Gynecol Oncol 2013;131:531-4. [Crossref] [PubMed]
- Petrillo M, Zannoni GF, Tortorella L, et al. Prognostic role and predictors of complete pathologic response to neoadjuvant chemotherapy in primary unresectable ovarian cancer. Am J Obstet Gynecol 2014;211:632.e1-8. [Crossref] [PubMed]
- Böhm S, Faruqi A, Said I, et al. Chemotherapy Response Score: Development and Validation of a System to Quantify Histopathologic Response to Neoadjuvant Chemotherapy in Tubo-Ovarian High-Grade Serous Carcinoma. J Clin Oncol 2015;33:2457-63. [Crossref] [PubMed]
- Coghlan E, Meniawy TM, Munro A, et al. Prognostic Role of Histological Tumor Regression in Patients Receiving Neoadjuvant Chemotherapy for High-Grade Serous Tubo-ovarian Carcinoma. Int J Gynecol Cancer 2017;27:708-13. [Crossref] [PubMed]
- Lee JY, Chung YS, Na K, et al. External validation of chemotherapy response score system for histopathological assessment of tumor regression after neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J Gynecol Oncol 2017;28:e73. [Crossref] [PubMed]
- Singh P, Kaushal V, Rai B, et al. The chemotherapy response score is a useful histological predictor of prognosis in high-grade serous carcinoma. Histopathology 2018;72:619-25. [Crossref] [PubMed]
- Ditzel HM, Strickland KC, Meserve EE, et al. Assessment of a Chemotherapy Response Score (CRS) System for Tubo-Ovarian High-Grade Serous Carcinoma (HGSC). Int J Gynecol Pathol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Gorodnova TV, Kotiv KB, Ivantsov AO, et al. Efficacy of Neoadjuvant Therapy with Cisplatin Plus Mitomycin C in BRCA1-Mutated Ovarian Cancer. Int J Gynecol Cancer 2018;28:1498-506. [Crossref] [PubMed]
- Leary A, Cowan R, Chi D, et al. Primary Surgery or Neoadjuvant Chemotherapy in Advanced Ovarian Cancer: The Debate Continues…. Am Soc Clin Oncol Educ Book 2016;35:153-62. [Crossref] [PubMed]
- Iyevleva AG, Imyanitov EN. Cytotoxic and targeted therapy for hereditary cancers. Hered Cancer Clin Pract 2016;14:17. [Crossref] [PubMed]
- International Collaboration on Cancer Reporting. Response to neoadjuvant therapy. Available online: [accessed 20 July 2018].https://www.rcpa.edu.au/Library/Practising-pathology/ICCR/docs/ICCR_Ovary_Response_neoadjuvant
- Chemotherapy response score. [Available online: ] [accessed 20 July 2018].http://www.gpecimage.ubc.ca/aperio/images/crs/
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 2017;376:2147-59. [Crossref] [PubMed]
- Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:3460-73. [Crossref] [PubMed]
- Sokolenko AP, Savonevich EL, Ivantsov AO, et al. Rapid selection of BRCA1-proficient tumor cells during neoadjuvant therapy for ovarian cancer in BRCA1 mutation carriers. Cancer Lett 2017;397:127-32. [Crossref] [PubMed]


