Systemic therapy for hepatocellular carcinoma: beyond sorafenib
Introduction
Hepatocellular carcinoma (HCC) remains the third leading cause of cancer mortality worldwide. In the United States, HCC is the fifth leading cause of cancer-related deaths (1,2). Even with advances in therapy, HCC still confers significant mortality, with 5-year survival rates of only 18% from 2005 to 2011 (2). In its early stages, surgery and liver transplantation are a potentially curative treatment (3). However, up to 80% of patients present at an incurable stage (4). In such situations, local therapies including transarterial chemoembolization (TACE) are often used to control the disease when HCC is confined to the liver. For those patients who progress or are not candidates for local treatments, systemic therapies are the only option (4).
Sorafenib, a tyrosine kinase inhibitor, was the first Food and Drug Administration (FDA) approved first line systemic therapy for HCC in 2007. Sorafenib provides multiple sites of action, inhibiting vascular endothelial growth factor receptor 1 (VEGFR1), VEGFR2, VEGFR3, platelet-derived growth factor receptor beta (PDGFR-β), and RAF-family kinases (5). In the landmark phase III SHARP trial including 602 HCC patients with mostly Child Pugh Class A, sorafenib improved median overall survival (mOS) compared to placebo (10.7 vs. 7.9 months; P<0.001) (6,7).
Even with modest improvement in survival, most patients have to be started on lower doses of sorafenib due to poor tolerance of side effects (8,9). It is clear that more effective and better-tolerated therapies are needed for this common and fatal disease.
Protein kinase inhibitors—anti-angiogenics
To date, there are 538 known protein kinases in the human genome (10-12). These proteins are responsible for the phosphorylation of serine, threonine, or tyrosine residues on proteins in the cell, greatly modifying their functions. Moreover, protein kinases are closely involved in the mutagenesis and growth of human cancer cells (13).
Up to now, 37 different kinase inhibitors have been FDA approved for the treatment of cancer, including sorafenib (14). Additionally, over a hundred new drugs are in clinical trials and hundreds more are incubating in the preclinical ecosystem of the biopharma industry. Among these, a significant number of agents beyond sorafenib have shown efficacy in the treatment of advanced HCC, all of which exhibit action against angiogenesis through inhibition of the VEGFR pathway (14).
First line therapy
Lenvatinib
Lenvatinib, the most notable of these to date, is a multikinase inhibitor which affects VEGFR1–3, fibroblast growth factor receptor (FGFR) 1–4, PDGFR-alpha, RET, and KIT (15). In the phase III randomized control trial REFLECT, researchers compared lenvatinib to sorafenib in the treatment of Child Pugh Class A unresectable HCC in a cohort of 954 patients. Lenvatinib met its primary endpoint of non-inferiority compared to sorafenib, with a mOS of 13.6 vs. 12.3 months respectively [hazard ratio (HR) 0.92, 95% confidence interval (CI): 0.79–1.06] (16). It also achieved the secondary endpoints of progression free survival (PFS) of 7.4 vs. 3.7 months (HR 0.66, 95% CI: 0.57–0.77) and time to progression (TTP) of 8.9 vs. 3.7 months (HR 0.63, 95% CI: 0.53–0.73). Most notably, lenvatinib conferred an objective response rate (ORR) of 24.1% compared to 9.2% in the sorafenib arm per investigator review according to modified RECIST (mRECIST). This is the only tyrosine kinase inhibitor that showed non-inferiority compared to sorafenib in the 1st line treatment of HCC, providing an option for such patients beyond sorafenib, and has been recently approved in Japan, United States, and China.
Second line therapy
Regorafenib
Regorafenib, another oral multikinase inhibitor, has a wide range of targets including VEFGR 1–3, tyrosine kinase with immunoglobulin and epidermal growth factor homology domain 2 (TIE-2), PDGFR-beta, c-KIT, RET, RAF-1, and BRAF (17,18). It was approved April 2017 by the FDA as a second line treatment in HCC patients previously treated on sorafenib. In the phase III RESORCE study comparing regorafenib to placebo with progression on sorafenib, the mOS for regorafenib vs placebo was 10.6 vs. 7.8 months respectively (HR 0.63, 95% CI: 0.50–0.79) (19). The median PFS was 3.1 vs. 1.5 months (HR 0.43, 95% CI: 0.35–0.52). Similar to sorafenib, regorafenib has a common side effect, hand-foot skin reaction. In the RESORCE trial, patients who had hand-foot skin reaction during cycle 1 of regorafenib had better mOS of 14.1 vs. 6.6 months compared to those without (20). Of note, only patients who could tolerate and progress on sorafenib were enrolled in RESORCE trial and median duration of therapy of prior sorafenib in this study was 7.8 months, making regorafenib a good choice for patients who could stay on sorafenib for a relatively long period of time.
Cabozantinib
Cabozantinib is a tyrosine kinase inhibitor which inhibits VEGFRs, MET, AXL, RET, KIT, and FLT3 (21). In the recently published phase III CELESTIAL trial comparing cabozantinib to placebo in advanced HCC after sorafenib failure, cabozantinib and placebo achieved a mOS of 10.2 vs. 8.0 months respectively (P=0.005) (21). The median PFS was 5.2 vs. 1.9 months respectively (P<0.001). However, cabozantinib was associated with grade 3 or 4 adverse events in 68% of patients compared to 36% with placebo. The most common side effects were palmar-plantar erythrodysesthesia, hypertension, elevated transaminases, fatigue, and diarrhea. In the wake of this study, cabozantinib is poised to be a second line treatment option for HCC, though with a risk of high-grade adverse events nearly double that of placebo.
Ramucirumab
Ramucirumab is a monoclonal antibody (IgG1) which selectively inhibits VEGFR2 (22). The phase III REACH study compared ramucirumab to placebo as second line treatment for 565 HCC patients with Child Pugh Class A. There was no significant change in mOS between ramucirumab and placebo (9.2 vs. 7.6 months respectively) (22). However, ramucirumab achieved a higher ORR than placebo (7% vs. <1% respectively). In the prespecified retrospective analysis of the 250 patients with an alpha-fetoprotein (AFP) ≥400 ng/mL, the mOS of ramucirumab was 7.8 months compared to 4.2 months of placebo. Thus, it is possible that AFP ≥400 could be a potential biomarker predicting response to ramucirumab therapy. A subsequent phase III study, REACH-2, explored the mOS difference between ramucirumab and placebo in 2nd line patients with AFP ≥400 ng/mL. This study was presented at ASCO 2018, indicating that ramucirumab improved mOS in patients who progressed on sorafenib with AFP ≥400 ng/mL (23). Of the 292 patients with Child-Pugh A, ramucirumab improved mOS from 7.3 months in the placebo arm to 8.5 months (HR 0.71, 95% CI: 0.53–0.95). The most common side effects of ramucirumab therapy were hypertension and fatigue.
Immunotherapy
It is well known that cancer utilizes multiple mechanisms to avoid detection by the immune system. One of these mechanisms is exerted through programmed death-ligand 1 (PD-L1), a ligand produced by tumor cells which binds to programmed death-1 (PD-1) receptor on T-cells (24). This interaction between PD-L1 and PD-1 inhibits T-cell activation and can lead to T-cell apoptosis. Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibits T-cell activation through induction of regulatory T cells (Tregs), which creates an immunosuppressive environment. It also binds B7 on antigen-presenting cells to inhibit T-cell activation (25,26).
Both of the above mechanisms are targets for a new class of immune checkpoint inhibitors, either through interruption of the interaction between PD-1 and PD-L1 or between CTLA-4 and B7. By interfering with cancer’s ability to ensure immune cell quiescence, these drugs can induce powerful immune responses to a diverse number of cancer, including melanoma, renal cell carcinoma, non-small cell lung cancers, and head and neck cancers (27-33). In fact, a number of these agents have shown significant promise in the treatment of advanced HCC.
Second line therapy
Nivolumab
Nivolumab, a PD-1 inhibitor, is currently approved as second-line systemic treatment in advanced HCC patients who progressed on prior sorafenib treatment. In the associated phase I/II CHECKMATE 040 trial, 48 patients were treated in the dose-escalation phase and 214 in the dose-expansion phase (34). The ORR was 20% in the dose-expansion phase and 15% in the dose-escalation phase. For the 214 patients in the phase II study, the OS with nivolumab at 9 months was 74%. Such high ORR and long OS rate at 9 months in 2nd line HCC sets a new standard of care, allowing more patients to be eligible for such therapy regardless of the duration of prior sorafenib therapy.
A subgroup analysis in this study showed that patients with a PD-L1 ≥1% achieved ORR of 26%, while patients PD-LA <1% showed ORR of 19%. Therefore, PD-L1 is not a predictive marker for response to PD-1 inhibitor therapy.
Finally, when researchers explored the subgroup of 56 patients who were sorafenib naïve, nivolumab showed ORR of 23% and OS rate of 82% at 9 months. This suggested that nivolumab might be beneficial as an alternative first line treatment of HCC patients in lieu of sorafenib. A phase III trial of nivolumab vs. sorafenib as first line treatment for advanced HCC has been completed and we are eagerly awaiting the results.
Pembrolizumab
Another anti-PD-1 inhibitor is pembrolizumab (35). In the phase II trial, Keynote-224, pembrolizumab was assessed in 104 advanced HCC patients with Child Pugh Class A who had progressed on prior sorafenib therapy. Pembrolizumab met the primary endpoint of an ORR of 17% (34). In the study, there was also a 6-month PFS of 73% and 6-month OS rate of 77.9%. These results suggested that pembrolizumab might be a promising new therapy for patients with advanced HCC after sorafenib failure.
Tremelimumab
Tremelimumab is an anti-CTLA-4 agent which has shown promise in the treatment of advanced HCC. In a phase II trial, tremelimumab was explored in 21 patients with hepatitis C virus (HCV)-related HCC that included both treatment naïve and previously treated patients with both Child-Pugh Class A and B. Among 17 evaluable patients, tremelimumab achieved an ORR of 17.6%, with mOS of 8.2 months (13). Notably, one of the patients also exhibited a decreased HCV viral load over the course of the trial, suggesting that tremelimumab could have a role in the treatment of HCV in addition to its actions against advanced HCC. Such an observation makes immunotherapy particularly compelling in viral hepatitis related HCC, with its potential of not only providing disease control for HCC but also viral load reduction for hepatitis C.
Combination therapies
In many other solid cancers, combination therapies of multiple systemic agents exhibit superior response and longer survival over single agents. However, these combination therapies also frequently come with increased toxicities. A number of trials combining multiple systemic treatment modalities are ongoing in advanced HCC.
In the phase I/II study of HCC patients treated with durvalumab (PD-L1 inhibitor) and tremelimumab (CTLA-4 inhibitor), 93% of patients had Child Pugh Class A and 30% were treatment naïve (36). Of these, a remarkable 20% achieved confirmed ORR. The subsequent phase III HIMALYA study is comparing durvalumab monotherapy, sorafenib, and the combination of tremelimumab and durvalumab. This study is being conducted as first line treatment in advanced HCC and we eagerly await the results.
Similarly, the anti-CTLA-4 agent, ipilimumab, is combined with the PD-1 inhibitor, nivolumab, in the fourth cohort of the Checkmate-040 trial (37). Combination therapies that target CTLA/PD-1 pathway have been successfully used in the treatment of advanced melanoma and advanced renal cell carcinoma, and are being explored in the treatment of non-small cell lung cancer (29,30,32).
In addition to combined immunotherapies, immunotherapy has been explored in combination with small molecular therapy. The rationale is that small molecules may help potentiate tumors to immunotherapeutic agents, either by priming the tumor microenvironment to allow better immune response, or by releasing antigens for immune recognition.
For example, at ASCO 2018, data was published with a combination of the PD-L1 antibody, atezolizumab, with bevacizumab, an anti-angiogenic agent. The study to date has a cohort of 26 patients with previously untreated, advanced HCC. In the 21 evaluable patients at the time of the presentation, confirmed partial responses occurred in 13 patients (63%). Calculations of PFS, TTP, and mOS are ongoing (38). Such impressive ORR suggests that atezolizumab plus bevacizumab in combination may be more efficacious than the immunotherapeutic agent on its own.
In another combination regimen, cabozantinib is currently being explored with various immunotherapeutic agents. In fact, the Checkmate-040 trial includes a cabozantinib combination cohort. This cohort is evaluating the tolerability and safety in patients a nivolumab plus cabozantinib doublet regimen or nivolumab-ipilimumab-cabozantinib triple-therapy.
Finally, there are early trials involving pembrolizumab therapy, pairing with either regorafenib or lenvatinib, respectively (25,39).
Conclusions
Hepatocellular carcinoma continues to confer significant morbidity and mortality in both the developed and the developing world. Since 2007, when sorafenib became the first systemic therapy approved by the FDA for treatment of advanced, unresectable HCC, a number of novel agents, including new protein kinase inhibitors, monoclonal antibodies, and new immunotherapeutic agents, have shown efficacy in the treatment of HCC (Table 1). Currently, lenvatinib, regorafenib and nivolumab are all FDA approved in the treatment of HCC. Cabozantinib, ramucirumab, and pembrolizumab are in the process of obtaining FDA approval for the treatment of HCC. Ramucirumab marks the first time in the treatment of HCC that biomarker-derived therapy has shown efficacy. It is exciting to see benefit for a patient population with poor prognosis with AFP ≥400 ng/mL. Further research into new agents, combination therapies, and the identification of the appropriate patient populations for each intervention will likely continue to improve outcomes for this otherwise devastating disease. There is a vast future in the systemic treatment of HCC, beyond sorafenib.
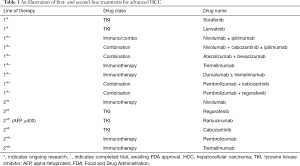
Full table
Acknowledgement
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J cancer 2015;136:E359-86. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Ulahannan SV, Duffy AG, McNeel TS, et al. Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatology 2014;60:1637-44. [Crossref] [PubMed]
- Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? Oncologist 2006;11:790-800. [Crossref] [PubMed]
- Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008;7:3129-40. [Crossref] [PubMed]
- Gauthier A, Ho M. Role of sorafenib in the treatment of advanced hepatocellular carcinoma: An update. Hepatol Res 2013;43:147-54. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Alghamdi MA, Lee-Ying R, Swiha M, et al. The effect of sorafenib (S) starting dose and dose intensity on survival in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2017;35:400. [Crossref]
- Kaplan DE, Yu S, Taddei TH, et al. Up-titration of sorafenib for hepatocellular carcinoma: Impact on duration of exposure and cost. J Clin Oncol 2017;35:385. [Crossref]
- Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576-96. [Crossref] [PubMed]
- Hunter T. Signaling--2000 and beyond. Cell 2000;100:113-27. [Crossref] [PubMed]
- Manning G, Whyte DB, Martinez R, et al. The Protein Kinase Complement of the Human Genome. Science 2002;298:1912-34. [Crossref] [PubMed]
- Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81-8. [Crossref] [PubMed]
- Bhullar KS, Lagarón NO, McGowan EM, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer 2018;17:48. [Crossref] [PubMed]
- Matsuki M, Adachi Y, Ozawa Y, et al. Targeting of tumor growth and angiogenesis underlies the enhanced antitumor activity of lenvatinib in combination with everolimus. Cancer Sci 2017;108:763-71. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (London, England) 2018;391:1163-73. [Crossref] [PubMed]
- Rey JB, Launay-Vacher V, Tournigand C. Regorafenib as a single-agent in the treatment of patients with gastrointestinal tumors: an overview for pharmacists. Target Oncol 2015;10:199-213. [Crossref] [PubMed]
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-55. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol 2018;36:412.
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63. [Crossref] [PubMed]
- Vennepureddy A, Singh P, Rastogi R, et al. Evolution of ramucirumab in the treatment of cancer – A review of literature. J Oncol Pharm Pract 2017;23:525-39. [Crossref] [PubMed]
- Zhu AX, Kang YK, Yen CJ, et al. REACH-2: A randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafe. J Clin Oncol 2018;36:4003. [Crossref]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Kudo M. Immune Checkpoint Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology 2017;92:50-62. [Crossref] [PubMed]
- Kudo M. Immuno-Oncology in Hepatocellular Carcinoma: 2017 Update. Oncology 2017;93:147-59. [Crossref] [PubMed]
- Santuray RT, Johnson DE, Grandis JR. New Therapies in Head and Neck Cancer. Trends in Cancer 2018;4:385-96. [Crossref] [PubMed]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-2104. [Crossref] [PubMed]
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378:1277-90. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017;377:1345-56. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [Crossref] [PubMed]
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol 2017;8:561. [Crossref] [PubMed]
- Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J Clin Oncol 2017;35:4073. [Crossref]
- Waidmann O. Recent developments with immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther 2018.905-10. [Crossref] [PubMed]
- Stein S, Pishvaian MJ, Lee MS, et al. Safety and clinical activity of 1L atezolizumab + bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC). J Clin Oncol 2018;36:4074. [Crossref]
- Ikeda M, Sung MW, Kudo M, et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol 2018;36:4076. [Crossref]


