
Valchlor maintenance therapy for patients with mycosis fungoides who received low dose total skin electron beam treatment
Introduction
Historically, radiation therapy achieves an extremely high response rate and functions as the single most effective modality in the treatment of mycosis fungoides (MF) (1). Total skin electron beam therapy (TSEBT) was introduced as treatment for patients with MF in 1952 with excellent responses and minimal toxicity (2). TSEBT treatment is appropriate for MF patients that have stage T2-4N0-1M0B0 disease at the time of initiation (1). Results from a study completed at Stanford University demonstrated that patients with T2 disease treated initially with TSEBT are more likely to achieve a complete response than those receiving nitrogen mustard alone. Patients treated with adjuvant nitrogen mustard following TSEBT maintained a higher freedom from relapse than those who achieved a complete response to low dose TSEBT. Out of the 148 subjects in a study 44% of subjects who received an initial treatment of low dose TSEBT followed by topical nitrogen mustard achieved a complete response (3).
This study will provide further evidence into the efficacy of topical nitrogen mustard [mechlorethamine (Valchlor)] as a treatment to increase duration of progression-free survival following treatment with TSEBT. This data will provide future physicians information on expectations and prognosis of Valchlor following TSEBT and efficacy of this regimen in comparison to other maintenance treatment modalities.
Case presentation
Patient 1
A 64-year-old African American female originally presented to the Jefferson multidisciplinary cutaneous lymphoma clinic (MCLC) in 2012 with a history of known MF for greater than 40 years. Upon physical exam, she was found to have generalized, violaceous, hyperpigmented, lichenified plaques and patches with underlying mottling on greater than 90% of her skin with erythematous, eroded plaques, nodules, and tumors and hyperkeratotic, exophytic, yellow plaques. She was also found to have palpable, non-tender lymphadenopathy in her bilateral axillary nodes and in her left inguinal nodes. She was treated with various modalities including: topical steroids, narrow band ultraviolet B (nb-UVB), romidepsin, pralatrexate and Valchlor. Despite these treatments, she had eventual progression of disease.
She received low dose TSEBT to better control overall disease. She had to stop at 10 Gray before completion of the total 12 Gray was completed due to side effects. A month following completion of treatment, she was instructed to apply Valchlor to all of her lesions once weekly in addition to topical steroid creams. Although instructed to increase her dosage to everyday over her entire body, she applied Valchlor approximately three times weekly. Full resolution was not achieved so nb-UVB was added. Progression was noted after 21 months despite admitting she stopped Valchlor treatment six months previously.
Patient 2
A 76-year-old Hispanic male presented to an outside physician with a one-year history of lesions originally diagnosed as peripheral T-cell lymphoma. He was started on cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and although initially responded well, continued having persistent lesions. After discontinuation of CHOP and subsequent return of lesions, he was referred to the Jefferson MCLC for further evaluation. On physical exam, he had patches, plaques and tumors over approximately 30% of his body.
Upon reevaluation, a diagnosis of MF was favored after another biopsy showed primary cutaneous T-cell lymphoma (CTCL). He was then given low dose TSEBT for rapid disease clearance. Clobetasol was initially given to him to maintain his clearance, but he started to have progressive disease. Valchlor was then added which was gradually increased to five applications weekly. One single fraction dose of X-ray therapy (XRT) was given for a lesion on his left arm and oral bexarotene was eventually added to his treatment regimen. Despite these therapies, he progressed to a greater stage after 24 months and was started on brentuximab.
Patient 3
A 37-year-old African American male presented for an evaluation of biopsy-proven MF from an outside dermatologist. Initially presenting with a widely distributed discolored and pruritic rash present for three years, a biopsy revealed nummular eczema and he started topical steroids with no improvement. Subsequent biopsies revealed MF and a dysplastic nevus exhibiting epidermotropic lymphocytes, both positive for TCR gene rearrangements. He was initiated on light therapy and referred to Jefferson’s MCLC.
On physical exam, he showed defined hyperpigmented round patches with overlying thin scale on his arms, thighs and trunk and thick adherent plate-like scale bilaterally on his lower extremity and upper back. His body surface area was determined to be approximately 20% and low dose TSEBT was determined to be an appropriate option for treatment.
Following low dose TSEBT, he was started on Valchlor gel three times weekly. He has been following this regimen since then with only minor breakthroughs. His body surface area (BSA) has increased slightly with one percent two months post-treatment and two percent five months post-treatment.
Patient 4
A 69-year-old Caucasian male presented with a history of MF in clinical remission following nb-UVB with recurrence for the last six months on his lower extremities. Physical exam showed pink thin plaques with fine scale on his bilateral lower extremities, lower abdomen, and suprapubic pelvis, a slightly indurated, hyperpigmented, slightly pink, thin plaque on his left thigh, and pink, well demarcated plaques with thick, dry scale on his feet. He was subsequently treated with topical steroids and antifungal cream for his feet.
After six weeks with no improvement, local radiation therapy (XRT) was utilized and allowed lesion improvement. Valchlor was then initiated once weekly building up to four times weekly. Although stable for five months, progression was noted and he received low dose TSEBT.
A month after low dose TSEBT, he began applying Valchlor once weekly to his entire body and was evaluated nine months later still within stage IA, with only slight increase in body surface area. No stage progression has been noted to date.
Patient 5
An 80-year-old male presented to the Jefferson MCLC with a 13-year history of biopsy-confirmed MF. Previously treated with light therapy and Valchlor, he had been untreated for the past year. On physical exam the day of presentation he was found to have erythematous, scaly, and indurated patches located on his lower abdomen, right hip and left buttock, an erythematous, indurated plaque on his left posterior knee, and a thick, hyperpigmented plaque with shallow fissures on the sole and lateral plantar aspect of his left foot. He was determined to have disease affecting 10–15% of his body surface area and due to his age and thickness of lesions was initiated on TSEBT. The patient received 12 Gray low dose TSEBT divided into 6 fractions with boosts to the perineum and bilateral soles of his feet.
One month after completing low dose TSEBT the patient was found to have improvement of his lesions with disease involvement affecting 5–8% body surface area. On physical exam, he was found to have reduction of thickness in all lesions and faint, pink scaly patches on his lower abdomen, right hip, left buttock, left posterior knee and a slightly erythematous patch on his left foot. He was determined to be in partial remission and was started on maintenance therapy with Valchlor. Twenty-six months since completion of his low dose TSEBT and maintenance with Valchlor therapy, the patient has not showed any recurrence of disease or increase in staging.
Patient 6
A 69-year-old female presented to the Jefferson MCLC after having biopsy biopsy confirmation. Although previously treated with Valchlor and oral bexarotene, she suffered from recalcitrant disease. At her primary visit she was found to have erythematous to brown, mildly scaly and slightly tender patches on her bilateral breasts, flanks, buttocks, lateral thighs, upper arms and abdomen and a BSA >10%. Low dose TSEBT was promptly initiated.
The Patient significantly improved following completion of low dose TSEBT. After her radiation dermatitis cleared, she was initiated on a regimen of Valchlor two times weekly. The patient continued to improve until she achieved complete remission and no recurrence of disease for the past 30 months has been noted.
Patient 7
A 61-year-old male hepatitis B carrier presented to the Jefferson MCLC with a 4-year history of biopsy confirmed folliculotropic MF. Presenting for a second opinion, he had previously received treatment with clobetasol, nitrogen mustard ointment and psoralen plus ultraviolet A (PUVA). On physical exam, he had alopecic patches on his dorsal hands and distal forearms, medial arms and forearms, flanks and hips, and patchy alopecic areas on his legs and lower legs. Folliculocentric hyperkeratosis was noted throughout his alopecic patches. On his bilateral hips, he was noted to have extensive deep boxcar and rolling scars, folliculocentric gray-brown plugging and confluent hyperpigmented patches. Additionally, he had confluent erythema with erythematous papules located on his occipital hairline and perineal area.
His BSA was measured to be approximately 60%. The patient was started on TSEBT and completed 12 Gray with 8 Gray boosts to the perineum, sole of foot and scalp over the duration of one month. With only a partial response to TSEBT and a subsequent BSA of 50% at one month follow-up visit, Valchlor therapy was initiated. The patient continued to improve on this regimen and over the following year decreased his BSA involvement to 10%. Despite treatment, the patient had disease recurrence 18 months after completion of TSEBT and was started on imiquimod in addition to Valchlor with a partial response and significant reduction of in MSWAT.
Patient 8
An 81-year-old male presented to the Jefferson MCLC after a 6-month history of biopsy-proven MF recalcitrant to nb-UVB. Physical exam noted thin, scaly plaques and patches with erythema on his back and bilateral forearms, flanks and thighs. He had multiple arcuate to polycyclic erythematous patches and intervening thin scaly plaques on both buttocks. Blood involvement was noted via flow cytometry, but was still considered B0 via National Comprehensive Cancer Network (NCCN) criteria. With a BSA 15–20%, he was continued on nb-UVB with the addition of clobetasol until the emergence of new lesions.
The patient then received 12 Gray of TSEBT over three fractions with boosts to the perineum and sole of the foot. A month after he completed his low dose TSEBT, he had <1% of his BSA in near complete remission. He was initiated on a regimen of Valchlor alternating with Clobetasol daily to MF lesions for maintenance therapy. The patient remained stable for 10 months until disease progression was noted with the presentation of scalp tumors. The patient was then started on brentuximab.
Results
Eight patients at the Jefferson MCLC were initiated on a regimen of Valchlor as a maintenance therapy after completion of TSEBT. Measures of disease severity were collected at the patient visit before TSEBT and after completion of low dose TSEBT to establish efficacy of TSEBT as a primary treatment modality. These measures were pain, pruritus, and quality of life score. Additionally, a modified severity weight assessment score (MSWAT) was collected. This score represents the severity of disease in regard to morphology and percent body surface area. Efficacy of low dose TSEBT as a primary treatment regimen was confirmed by reductions across all measures with the exception of pain. The median MSWAT score before TSEBT was found to be 25.25 with a mean of 39.76. A reduction was found in MSWAT score after TSEBT to a median of 7.68 and a mean of 17.31. Furthermore, median scores for pruritus were decreased from 3.43 before TSEBT to 1.88 after TSEBT and a decreased in quality of life score median from 6.60 to a median of 2.75 was found (Tables 1,2).
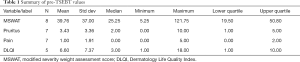
Full table
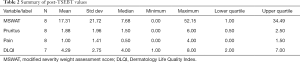
Full table
The primary endpoint for our study was stage progression in patients initiated on a regimen of Valchlor as a maintenance therapy after completing low dose TSEBT. Results demonstrated a median time to progression of 22.7351 months (95% CI: 10.7105–24.7392). Further analysis demonstrated that 75% of our population progressed by 24.7392 months and 25% of our patient population progressed by 22.7351 months (Table 3).
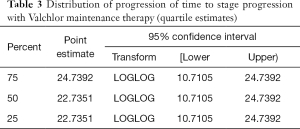
Full table
Discussion
Valchlor (mechlorethamine) 0.016% gel is a topical chemotherapeutic agent used for the treatment of MF, the most common type of CTCL. MF is characterized by a neoplastic proliferation of T-cells within any layer of the skin. Mechlorethamine gel functions as an antineoplastic therapy via three mechanisms: attachment of alkyl groups to DNA bases, resulting in fragmentation and cell death, DNA damage via the formation of cross-links, and induction of mispairing of nucleotides leading to mutations (4). Previous literature has demonstrated the efficacy of Valchlor as a primary treatment modality, but no literature has been established evaluating its efficacy as a maintenance therapy after completion of low dose TSEBT. Currently, there are no guidelines that exist for therapeutic modalities in regard to maintenance therapy after the completion of total body radiation. Our clinic has found useful, in select patient scenarios, in using Valchlor as maintenance therapy. We believe that when compared to other therapies, it is effective in preventing recurrence of disease in the appropriate clinical setting. The specific patients that we select to use Valchlor in the post-TSEBT setting lack significant blood involvement (B0), may have a poor performance status, are unable to tolerate systemic medications or infusions, fail to qualify for insurance purposes, are elderly patients, or are patients in need of rapid symptomatic relief. In addition, for our patients working full-time, low dose TSEBT requires only 2 weeks off of work followed then by maintenance Valchlor applied at home.
Evaluation of eight patients in our study found a median time to progression of 22.7 months. Time to disease progression following high dose TSEBT in all disease stages has been reported in multiple studies in the literature. Becker et al. reported a mean duration of complete response of 11.6 months following the first course of high dose TSEBT utilizing between 25-36 Gray. (5). Additionally, a study conducted at Yale University demonstrated a median disease-free interval after the first course of high dose TSEBT (36 Gray) for those patients with a complete response to be 20 months (6). A French study evaluated the treatment results of 57 patients that received radiotherapy. This study found that 54.4% of the patients that received high dose TSEBT at 24–30 Gray had skin failure within one year (7). Our study demonstrated a median time to progression of 22.7 months. Progression was defined as an increase in staging. It is important to note that the results demonstrated in our study utilizing low dose TSEBT (12 Gray) as the primary treatment modality are comparable to the results seen in previous studies which all used high dose regimens. Compared to patients in previous studies, the patients who received low dose TSEBT with maintenance Valchlor were documented to have a clinically significant longer time period to disease progression. We believe that the efficacy of Valchlor as a maintenance therapy is both due to the intrinsic properties and our patient instruction and monitoring model leading to higher than average compliance.
At the Jefferson MCLC, Valchlor is commonly used as a maintenance therapy, and we believe that specific care to ensure patient compliance and understanding of both the application of the therapy and the side-effect profile make our patient population more successful with this treatment as a maintenance therapy. In order to ensure patients understand timing of application, each patient is provided with a take home checklist that documents their specific regimen. They also receive a demonstration in applying the drug by a medical assistant utilizing a sample vehicle tube. Additionally, the patient is informed of the common side effects of the drug, most commonly being irritant dermatitis, and is told to return to clinic for follow up when this side effect is most likely to arise (one month after use). This allows the provider to treat the dermatitis and keep the patient compliant and on the appropriate regimen.
After completing low dose TSEBT, patients are brought back in one month. If there are no signs of severe radiation dermatitis or rapid disease progression, patients are initiated on Valchlor maintenance. Given the results of this study, we recommend patients that have undergone low dose TSEBT should receive Valchlor therapy as a maintenance therapy until progression of disease is noted or adverse effects of the medication cause the patient to discontinue therapy.
Due to the limited number of patients in this cohort, it is difficult to expand our conclusions to the patients diagnosed with MF as a whole. Additionally, many of our patients presented to the clinic at differing stages and analysis was not completed individually based on initial stage. As this study group increases in our patient population, further studies can be conducted stratifying the results based on initial stage. Additionally, our results can be further verified by continuing to follow the patient population with regards to their survival rates as many previous studies evaluate the efficacy of low dose TSEBT based on 5-year survival rates. The 5-year survival rates in this patient population can then be correlated to the previous studies evaluating the use of high dose TSEBT as a monotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The Jefferson IRB approved this retrospective review and waiver of informed consent. This study is in full compliance with our institution.
References
- Harrison C, Young J, Navi D, et al. Revisiting low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncol Biol Phys 2011;81:e651-7. [Crossref] [PubMed]
- Lo TC, Salzman FA, Moschella SL, et al. Whole body surface electron irradiation in the treatment of mycosis fungoides. An evaluation of 200 patients. Radiology 1979;130:453-7. [Crossref] [PubMed]
- Chinn DM, Chow S, Kim YH, et al. Total skin electron beam therapy with or without adjuvant topical nitrogen mustard or nitrogen mustard alone as initial treatment of T2 and T3 mycosis fungoides. Int J Radiat Oncol Biol Phys 1999;43:951-8. [Crossref] [PubMed]
- Povirk LF, Shuker DE. DNA damage and mutagenesis induced by nitrogen mustards. Mutat Res 1994;318:205-26. [Crossref] [PubMed]
- Becker M, Hoppe RT, Knox SJ. Multiple courses of high-dose total skin electron beam therapy in the management of mycosis fungoides. Int J Radiat Oncol Biol Phys 1995;32:1445-9. [Crossref] [PubMed]
- Wilson LD, Quiros PA, Kolenik SA, et al. Additional courses of total skin electron beam therapy in the treatment of patients with recurrent cutaneous T-cell lymphoma. J Am Acad Dermatol 1996;35:69-73. [Crossref] [PubMed]
- Ysebaert L, Truc G, Dalac S, et al. Ultimate results of radiation therapy for T1-T2 mycosis fungoides (including reirradiation). Int J Radiat Oncol Biol Phys 2004;58:1128-34. [Crossref] [PubMed]


