
Systemic therapy of cutaneous T-cell lymphoma (CTCL)
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a heterogeneous subset of extranodal non-Hodgkin lymphomas (NHL) of mature, skin-homing T-cells that are mainly localized to the skin. The most common types of CTCL are mycosis fungoides (MF) and primary cutaneous CD30+ anaplastic large cell lymphoma (pcALCL), jointly representing an estimated 80–85% of all CTCL. Sézary Syndrome (SS), a very rare subtype (~2–5% of CTCL) characterized by diffuse inflammatory, often exfoliative, erythroderma and by leukemic and nodal involvement, displays a significant degree of clinical and biological overlap with MF and has long been considered a clinical variant of MF, although recent evidence suggests that it may be a separate entity. The rest is represented by extremely rare, generally more aggressive subtypes. In light of the overlap between MF and SS, and considering that many of the systemic therapy options for the two neoplasms are the same, this review will discuss the treatment approach to MF and SS as if they were a single disease entity (MF/SS). However, some of the drugs currently in use, or in development, for MF/SS appear to be more effective in clearing different anatomical compartment (skin versus blood, for example) and therefore have differential efficacy in MF and SS.
Based on Surveillance Epidemiology and End Results (SEER) data from 2001–2007, the estimated incidence rate of MF/SS in the United States (US) is 0.5/100,000 or about 2,500–3,000 new cases per year representing about 25% of all T-cell lymphomas. The incidence of MF/SS has increased in the US from 1973 to 2002 (1). The cause of this increase is not clearly known; however, this may partially represent a greater degree of awareness and reporting of the disease.
Unlike other forms of NHL, which can involve the skin as a secondary extranodal site, the main feature of MF is that the skin is the primary site of involvement at diagnosis, and the disease can remain limited to the skin for the entire lifespan of the patient. MF/SS is staged according to four anatomical compartments: involvement of skin (T), nodes (N), visceral metastases (M) and peripheral blood (B) (Tables 1 and 2). In about two thirds of the patients the disease presents with skin-only involvement with patch and plaque type lesions, defined as early stage MF (T1, T2). About one third of the patients presents with skin tumors, or advanced cutaneous stage MF (T3), and a small minority presents with generalized (>80% BSA) erythroderma (T4) and high tumor burden in the blood (B2), with or without nodal (N) and visceral (M) disease, which are the hallmark of SS (3,4). At this time, stage remains the most significant prognostic factor in patients with CTCL (5,6). Subjects with early stage MF have a median survival in excess of 25 years; the median survival for patients with advanced stage MF and SS is very variable, with some studies reporting survival as short as 1.5 years (5).

Full table
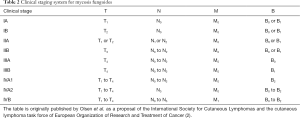
Full table
Systemic treatment of CTCLs
Overview
Patients with early-stage disease are treated with skin-directed therapies as discussed elsewhere in this issue. As the disease progresses, systemic therapy may become necessary. There is no standard systemic therapy for patients with advanced stage MF and SS. Both the Food and Drug Administration (FDA) approved, and unapproved agents are used in these patients including immune modulators, antibodies, single agent or combination chemotherapy, or other investigational agents. Current guidelines by the National Comprehensive Cancer Network (NCCN) recommend a variety of medications or a clinical trial as a first-line therapy. There are six FDA approved agents in the US for the treatment of CTCL: bexarotene, vorinostat, denileukin diftitox (DD), romidepsin, brentuximab and most recently mogamulizumab. DD has not been available in the US since 2014. All of these agents are indicated only after patients have failed at least one other systemic therapy.
The goals of systemic therapy
The main goals of treatment in patients requiring systemic therapy are long-term disease control, effective symptom management, and control of life-threatening complications (7). Generally, patients with very early stage (stage IA) do not need systemic therapy and their disease and symptoms can be controlled well with skin-directed therapy only (SDT) (8). On the other hand, some patients with early stage disease with more extensive skin involvement (stage IB) will fail SDT and need to start systemic therapy (5,7). Median survival decreases as skin stage progresses (5,9). Therefore, the goal for the systemic therapy for the patients with early stage disease is to prevent progression to higher stages and achieve a durable response. Patients with more advanced stage disease (stage IIB–IV) will require systemic therapy to control symptoms and achieve disease control. Patients with the most advanced stages (stage IVA and IVB), those with bulky, diffuse lymphadenopathy, elevated lactic dehydrogenase, high circulating tumor burden, and visceral disease, however, have very poor survival, regardless of systemic therapy (9-11). Therefore, there is a need to develop new drugs that can target all disease compartments (skin, blood, lymph nodes, and viscera), provide more durable responses, and improve the poor outcomes of patients with advanced stage MF and SS. In this review, we will discuss each systemic therapy according to their mechanism of action, and discuss the indications and the sequence, in the patients with CTCL.
The drugs that can be used in the systemic therapy of CTCL (Table 3)

Full table
Biologic response modifiers (BRMs)
Retinoids
Bexarotene
Bexarotene is a synthetic retinoid that belongs to a group of compounds called rexinoids because they selectively activate retinoid-X receptors (RXRs). These retinoid receptors have biologic activity distinct from that of retinoid acid receptors (RAR). The RXR interacts as a heterodimer with other nuclear receptors including those controlling lipids, insulin sensitivity, drug metabolism, bile acid metabolism, and liver lipid metabolism. RXR also interacts with RAR, vitamin D, glucocorticoid, and sex steroid receptors (12). Bexarotene regulates multiple cellular processes including cellular differentiation, proliferation, apoptosis and insulin sensitization by binding and activating RXR-α, -β, and -γ. Bexarotene was shown to induce tumor cell apoptosis in a variety of preclinical models including CTCL cell lines. Nieto-Rementeria and colleagues reported that bexarotene decreases the viability of human CTCL cells by activating the p53/p73 pathway (13). This effect of bexarotene is mediated by upstream ataxia telangiectasia mutated protein (ATM) activation (13).
Bexarotene was approved by FDA in 1999, for use in patients with advanced stage of MF who are refractory to at least one prior systemic therapy. European Medicines Agency’s (EMA) also approved bexarotene for the same indication in early 2001. Duvic and colleagues showed that oral bexarotene is an effective therapy for patients with CTCL in a multi-center clinical study. Ninety-four patients with CTCL in advanced stages (IIB–IVB) were enrolled in the study. Fifty-six patients received an initial dose of 300 mg/m2/day oral bexarotene, and 38 started at doses greater than 300 mg/m2/day. Overall clinical response rate (CR + PR) was 45% in the first group and 55% in the patients who received more than 300 mg/m2/day (14). Bexarotene showed a similar efficacy in patients with early stage of CTCL (ORR of 54%) (15). Bexarotene is generally well tolerated with reversible side effects (14). The most frequent drug-related adverse events (AEs) were hyperlipidemia primarily hypertriglyceridemia (82%), hypercholesterolemia (30%), hypothyroidism (29%), headache (20%) asthenia (16%), pruritus (13%), leukopenia (11%), and rash (11%).
The recommended treatment dose of bexarotene is 200 or 300 mg/m2/daily. In most cases patients are started on a low dose, such as 150 mg/daily, and then titrated up to 300 mg/m2, depending on AEs and patient tolerance. Complete blood counts (CBC), fasting lipid profile, thyroid hormone levels (free T4), and liver function tests should be ordered before starting bexarotene and monitored during the treatment. Using lipid-lowering agents with bexarotene may increase tolerance to the medication and permit the use of higher doses (16).
Bexarotene has also been studied, and is often used in combination with other systemic treatments including interferon-α (IFN-α), methotrexate (MTX), DD, gemcitabine, HDAC inhibitors, and pralatrexate to enhance treatment efficacy in CTCL (16-22). However, due to the lack of well-designed, large randomized, prospective studies, the advantage of combination therapy with bexarotene versus its use as a single agent has not been clearly defined. Bexarotene is often used in combination with skin-directed therapies including topical corticosteroids, topical nitrogen mustard, light therapy and/or radiation therapy. Selected studies with bexarotene in CTCL are shown in Table 4.

Full table
Other retinoids (isotretinoin and acitretin) are commercially available in the US, but not FDA-approved for this indication. Etretinate is available only in Japan.
Interferons (IFNs)
IFNs are naturally produced polypeptides as a part of the innate immune system with anti-viral, immune modulatory and cytostatic activities. There are three types of IFNs; IFN-α, interferon-beta (IFN-β) and interferon-gamma (IFN-γ) . The activity of IFNs in CTCL has recently been reviewed elsewhere in detail (24).
IFN-α
IFN-α’s immunomodulatory activity plays a critical role in its activity in MF/SS. Tumor cells from patients with MF/SS produce T-helper 2 (Th2) type cytokines including IL-4, IL-5, and IL-10 (24-26). IFN-α activates CD8+ T cells and NK cells (27) and suppresses Th2 cytokine production from malignant lymphoma cells, which results in the improvement of Th1/Th2 balance. IFN-α has been used for the treatment of patients with CTCL and specifically MF/SS since the early 1980s (28,29). High dose recombinant leukocyte A IFN was tested in twenty patients with advanced CTCL at a dose of 50 MU 3 times weekly. All 20 patients had advanced stages of disease refractory to two or more standard therapies. Objective responses were observed in 9 patients with a median duration response of 5 months (28). Because of the toxicities at these high doses, Kohn et al. investigated the role of pulse IFN dosing in patients with CTCL (30). Twenty-four patients with advanced disease refractory to one or more standard therapies received IFN-α, 10 MU/m2 IM on day 1 followed by 50 MU/m2 IM on days 2 to 5, every three weeks. The ORR was 29% with 1 CR and 6 PRs. They observed that intermittent high-dose IFN-α was better tolerated.
IFN-α therapy resulted in a high response rate in previously untreated patients (31). Twenty-eight newly diagnosed and 15 previously treated patients were treated. IFN was given daily with dose escalation from 3 to 18 MU. Ninety percent of previously untreated patients who obtained a CR remain disease free after 18 to 40 months. Jumbou and colleagues retrospectively evaluated 51 patients with CTCL with a long-term follow up (32). All patients received low-dose IFN-α (mean daily dose 2.7 MU) for 14.9 months. This resulted in CR in 21 of 51 patients (41%). However, 57% of the patients relapsed at a mean period of 7.5 months, independent of their stage.
More recently, Hughes and colleagues retrospectively analyzed outcomes in 198 patients who received a variety of systemic therapies. Twenty-eight different treatment modalities were analyzed. The median time to next treatment (TTNT) for single- or multi-agent chemotherapy was found to be only 3.9 months, with few durable remissions while IFN-α had a significantly better TTNT to 8.7 months (33).
Several studies have examined the use of IFN-α in combination with other therapeutic modalities to improve efficacy. PUVA and IFN-α combination (with varying treatment protocols) resulted in a CR rate of 45% to 84% in patients with CTCL (34-39). A robust efficacy for the combination of IFN-α and extracorporeal photopheresis (ECP) has been reported, in both retrospective and prospective studies (40,41). Investigators from the University of Pennsylvania showed that the combination with ECP and immune modulatory agents including IFN-α could provide high overall response (OR) and CR rates in patients with SS (42). On the other hand, adding IFN-α to total skin electron beam therapy (TSEBT) compared to TSEBT alone, did not result in a significant difference in CR, DFS, and OS (43). Wagner and colleagues retrospectively analyzed 41 patients with CTCL who received either TSEBT alone (n=11) or TSEBT and IFN-α (N=30) (44). They observed a CR rate of 63% in the combination group compared to 35% in TSEBT group. However, the difference was not statistically significant.
Strauss and colleagues explored the efficacy of bexarotene and IFN-α combination in patients with CTCL (17). Twenty-two patients with stage I–IV were first treated with bexarotene for eight weeks. The patients who did not have a response to bexarotene were treated with IFN-α and bexarotene combination. Three out of 8 patients developed a response after adding IFN-α.
IFN-α was also studied in combination with chemotherapy including pentostatin (45), fludarabine (46), and MTX (47). The combination of MTX and IFN-α resulted in impressive outcomes in a large group of patients. One hundred fifty eight patients with advanced stage (stage IIB–IV disease) CTCL were evaluated. MTX was given 10 mg/m2 twice weekly in combination with IFN-α 9 MU three times a week for six months (47). The study showed a very high clinical efficacy with a CR 74% and 10-year estimated survival of 69%. Treatment was tolerated well without significant toxicity. Although some IFN-α combinations with other modalities resulted in promising results in CTCL treatment, the superiority of any IFN-α combination has not been proven yet, in light of the lack of randomized studies. Selected studies with IFN-α are shown in Table 5.
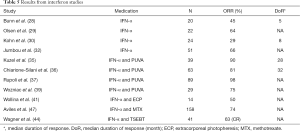
Full table
As discussed above, IFN-α is generally tolerated well with mostly tolerable and reversible toxicities. Most common side effects are flu-like symptoms such as fevers, fatigue, headache chills, myalgias, and arthralgias. Other less common side effects are weight loss, cytopenia, LFT abnormalities, hypothyroidism, depressive mood, impaired cognitive function, and cardiac side effects.
IFN-γ
IFN-γ is an immunologically active cytokine, essential for Th1 immune response (48). IFN-γ can enhance both innate and adaptive immune response. IFN-γ has activity in CTCL. A phase I study showed a tolerable toxicity profile (49). Fever and flu-like symptoms were the most common side effects and were seen at all dose levels. Sixteen patients with CTCL were treated with IFN-γ. ORR was 31% with no CR (50). Dummer and colleagues evaluated the activity of intratumoral IFN-γ administration in patients with CTCL. They observed 5 ORs out of the 9 treated patients with cutaneous lymphoma (51). Three patients showed systemic CR with clearance of other non-injected skin lesions. Recently, IFN-γ was reported with a high ORR (11 out of 15 patients) in CTCL patients from Japan (52). Again no CR was observed.
Folic acid metabolism inhibitors
MTX
MTX is a well-known antimetabolite that blocks the cell division in the S phase. MTX targets explicitly the folic acid metabolism by competitively inhibiting the dihydrofolate reductase (DHFR) enzyme. DHFR converts dihydrofolate to tetrahydrofolate, which is required for DNA and RNA synthesis [reviewed in details (53)]. Low dose oral MTX (10–50 mg/weekly) is effective for the treatment of MF (54,55). Zackheim and colleagues retrospectively analyzed the efficacy of low dose MTX in patients with MF and 69 patients with patch/plaque and tumor stage MF were observed for up to 16 years. ORR in patients with patch and plague disease was reported as 33% with a median time to treatment failure of 15 months. However, only 1 out of 7 patients with tumor stage disease responded (55). MTX can be combined with other systemic therapy such as IFNs and bexarotene or skin-directed therapy; light therapy etc. MTX and IFN-α combination resulted in a very high clinical efficiency, and durable response (47). Treatment was tolerated well without significant toxicity.
MTX is also an effective therapy for lymphomatoid papulosis (LyP) and other primary cutaneous CD30-positive lymphoproliferative disorders (56). The expert consensus of the EORTC, ISCL, and USCLC recommends low-dose MTX for the treatment of primary cutaneous CD30 (+) lymphoproliferative disease, including LyP and pcALCL (57). Of course, in the Brentuximab era, the role of MTX for the treatment of CD30 (+) LPDs is being re-evaluated.
Common side effects of MTX include mucositis, myelosuppression; neutropenia and thrombocytopenia, gastrointestinal symptoms, liver function tests abnormalities (transaminitis), and pulmonary toxicity. In most cases MTX is started at a low dose (10–15 mg/weekly) and titrated the dose is slowly titrated to a higher dose level.
Pralatrexate
Pralatrexate is a synthetic folate analog and a member of 10-deazaaminopterin anti-folates that demonstrated greater anti-tumor effects than MTX in pre-clinical models (58). Pralatrexate inhibits folate metabolism by binding and inhibiting the enzyme DHFR. It has a higher affinity to reduced folate carrier (RFC) and folylpolyglutamate synthase (FPGS), resulting in increased intracellular internalization and retention in tumor cells (59). It is incorporated at a rate of nearly 14 times greater than MTX (60). Similarly, pralatrexate is also ten times more efficiently polyglutamylated than MTX. These biochemical features suggest that pralatrexate should be a more potent antineoplastic agent in comparison to MTX. Preclinical studies with lymphoma lines consistently demonstrated pralatrexate to be superior to MTX, and its activity correlates with tumor reduced folate carrier (RFC-1) gene expression (61).
Pralatrexate is an active agent in patients with T cell lymphoma (62-64) and was FDA-approved for PTCL in 2009. However, the ORR with pralatrexate in patients with relapsed and resistant systemic T cell lymphoma remains unsatisfactory. In PROPEL study, 111 patients with relapsed or refractory peripheral T-cell lymphoma (PTCL) were treated with pralatrexate, 30 mg/m2/week for 6 weeks in 7-week cycles. The response rate in 109 evaluable patients was 29% (32 of 109), including 12 complete responses (CR) (11%) and 20 partial responses (PR) (18%), with a median duration of response of 10.1 months. Median PFS and OS were 3.5 and 14.5 months, respectively. Foss and colleagues reported a subgroup analysis of PROPEL study that pralatrexate was effective in patients with transformed MF (tMF) (65). Twenteen patients with tMF were treated with a median of 10 pralatrexate infusions (starting dose of 30 mg/m2) administered weekly for six weeks in a 7-week cycle. Objective response rate in this subgroup was 25% (n=3) per independent central review and 58% per investigator assessment. However, the median duration of response was only 2.2 months. Pralatrexate might be a treatment option for tMF. Horwitz et al. conducted a study to find an effective and safer dose for the treatment of patients with CTCL (63). First, they found a dose with the de-escalation method that administered 15 mg, weekly for three weeks as an effective dose with a limited toxicity profile. Then, they studied the dose in the expansion cohort and found that the ORR was 45% after four cycles of therapy. Talpur and colleagues investigated the efficacy of pralatrexate, as a single-agent or combination with bexarotene, in the patients with refractory and relapsed MF (22). Twenty-six patients were enrolled in the study; 12 received pralatrexate and 14 received pralatrexate plus bexarotene. The ORR was 33% in the pralatrexate group and 50% in the combination group. Both groups tolerated the treatment well with acceptable side effects.
Pralatrexate is not FDA-approved for CTCL, but it is recommended by the NCCN as an option for systemic therapy in CTCL patients with relapsed and refractory disease. Most frequently pralatrexate is used in CTCL patients at a dose of 15 mg/m2 for three out of four weeks along with folic acid 1 mg by mouth daily and vitamin B12 injections every other month (63).
Histone deacetylase (HDAC) inhibitors
HDACs are enzymes involved in the remodelling of chromatin and have a key role in the epigenetic regulation of gene expression. The removal of acetyl groups from lysine residues on histone proteins by HDAC causes greater chromatin condensation and decreases accessibility by transcription factors, which controls transcriptional programs involved in cell survival, apoptosis and differentiation (66). HDAC also target nonhistone molecules that regulate cellular homeostasis (67). Histone deacetylation plays a role in the development of various cancers, generally associated with silencing tumor suppressor genes. The inhibition of the enzymatic activity of HDAC therefore, has emerged as a compelling strategy for cancer therapy (67,68).
HDAC inhibitors play an important role in the treatment of CTCL and PTCL (66,69). Three HDAC inhibitors have been FDA approved in the US for T cell lymphoma therapy including romidepsin in PTCL and CTCL, vorinostat in CTCL, and belinostat in PTCL. We will review specifically the activity and efficacy of vorinostat and romidepsin in patients with CTCL.
Vorinostat
Vorinostat induces cell cycle arrest, apoptosis, and antitumor activity in preclinical cancer models (70,71). Phase-I studies with oral and IV formulations of vorinostat showed tolerable toxicities, including fatigue, dehydration, diarrhea, nausea, vomiting, and thrombocytopenia (72,73). Duvic and coworkers conducted a phase II study to evaluate the activity of vorinostat in patients with refractory CTCL (74). The study confirmed the activity of vorinostat in CTCL with ORR rate of 31%. The vorinostat dose of 400 mg once daily was safe and effective in the patients. The efficacy of vorinostat was also tested in a multicenter, open-label, phase IIb clinical trial in patients with resistant/relapsed CTCL (75). Vorinostat was given orally at 400 mg once daily. Again responses were observed in 22 out of 74 patients (30%). CR was reported only in one patient. Ten out of 33 patients with SS responded. Duration of response was around six months. The common side effects are fatigue, diarrhea, nausea, thrombocytopenia, anorexia, taste abnormalities, weight loss, and muscle spasms. Based on these phase II studies, vorinostat was approved by FDA in 2006 for the treatment of patients with CTCL who received at least two prior systemic therapies. Vorinostat is the only available oral HDAC inhibitor and continues to have a role in the treatment of recurrent/resistant CTCL.
Romidepsin
Romidepsin is a potent class I-selective HDAC inhibitor, which was approved by the FDA in 2009 for patients with CTCL who have received at least one prior systemic therapy. Approval of romidepsin for CTCL was based on two phase-II studies. Whittaker et al. investigated the efficacy of romidepsin in patients with stage IB–IV refractory CTCL in a multicenter clinical study (76). Ninety-six patients were enrolled. Romidepsin was administered at 14 mg/m2 on days 1, 8 and 15 of a 28-day cycle for up to six cycles. The dose of romidepsin could be reduced in a response to AEs. The overall response rate (ORR) was 34%. The patients with advanced stage disease (stage IIB–IV) had a similar response rate (38%) including five CR. Median duration of response was 15 months. Generally, treatment was well tolerated with mild side effects including GI disturbances and asthenia. Another multi-institutional study for patients with refractory CTCL in stage IA-IV was conducted by the NCI (77). Romidepsin was administered as a 4-hour infusion at 18 mg/m2 on days 1 and 5 of a 21-day cycle for the first three patients. Then the dose was adjusted by amendment, and the patients were treated on the more tolerable schedule of 14 mg/m2 on days 1, 8, and 15 of a 28-day cycle. Doses were reduced from 14.0 to 10.5 mg/m2 or from 10.5 to 8.0 mg/m2 for toxicity. Dose escalation to 17.5 mg/m2 was allowed in the absence of toxicity. Seventy-one patients were included in the analysis. These patients received a median of four prior treatments, and 62 patients (87%) had advanced-stage disease. The ORR was 34% with 4 CR (6%). The median duration of response was 13.7 months. Both trials showed that romidepsin has activity in patients with relapsed/refractory CTCL with significant and durable responses. Duvic and colleagues found that romidepsin had a similar ORR in the patients with previous systemic chemotherapy without increasing toxicity (78). Seventy-three out of 96 patients in pivotal trial and 52 out of 84 patients in the NCI trial received previous systemic chemotherapy. ORRs in those patient populations were similar in both clinical trials (34.2 and 34.6 in the Pivotal and the NCI trial respectively). Median DOR in the Pivotal trial was 15.0 months and was 23.0 months in the NCI trial (78). The most common side effects of romidepsin are nausea vomiting, fatigue, and myelosuppression. Romidepsin may cause QT interval changes without significant change in LV function or myocardial damage (79). QT intervals should be monitored with serial ECGs especially in patients with, previous cardiac conditions and taking anti-arrhythmic medications that may cause QT prolongation. Serum magnesium and potassium levels should be monitored and kept within normal levels prior to administration of romidepsin.
Antibodies
Three antibodies are available for CTCL therapy; brentuximab vedotin, mogamulizumab and alemtuzumab. Brentuximab vedotin and mogamulizumab have been recently approved by FDA for patients with relapsed/refractory CTCL.
Brentuximab vedotin
BV is a CD30 specific antibody-drug conjugate (ADC), which consists of a chimeric IgG1 antibody, specific for human CD30 linked to the microtubule-disrupting agent monomethyl auristatin E (MMAE) via a protease-cleavable linker. The linker is a valine-citrulline dipeptide specifically designed for high stability in serum and conditional cleavage and release of fully active drug (80). BV is internalized by endocytosis after binding to the CD30 molecule, subsequently migrating to the lysosome where proteases cleave the linker peptide and release MMAE into the cytosol.
In light of its unique mechanism with high specificity for the CD30 molecule, BV is an ideal treatment for CD30 expressing malignancies. The CD30 molecule was first found to be highly expressed on Reed-Sternberg cells in Hodgkin lymphoma (81). CD30 was also found on anaplastic large cell lymphoma (ALCL) and other subtypes of NHL [reviewed in details (82,83)]. CD30 is also expressed on normal activated T and B cells, but not in other normal lymphocytes.
A Phase-I study with brentuximab vedotin in patients with CD30 (+) lymphomas initially established the maximum tolerated dose at 1.8 mg/kg every three weeks (84). The FDA later approved BV for the treatment of relapsed/resistant Hodgkin lymphoma and ALCL in 2011. Two phase-II clinical trials investigated the efficacy of BV in patients with CTCL (85,86). In the first study, Kim et al. treated 32 patients with MF and SS with variable CD30 expression, every three weeks for a maximum 16 cycles. ORR was 70%. In addition, there was no significant difference in response rates between early and advanced stages. Peripheral neuropathy was the most common side effects. The patients with less than 5% CD30 expression had a lower likelihood of OR than did those with 5% or greater CD30 expression (85). Duvic et al. evaluated the efficacy and safety of BV in 48 patients with the same dose and treatment schedule. ORR was 73% with CR of 35%. The median duration of response was 32 weeks. MF/SS patients with different CD30 expression (low, moderate and high) had a similar response rate (50–58%). All patients with LyP and pcALCL responded. Grade 1 and grade 2 neuropathy developed in 65% of the patients. Investigators concluded that BV is active and well-tolerated in patients with CTCL and lymphomatoid papulosis (86).
Recently a phase III multi-center clinical trial was conducted to compare the activity of BV with physician choice (oral MTX or oral bexarotene) in patients with CD30 (+) MF and pcALCL (87). Patients with MF had received at least one prior systemic therapy and those with pcALCL were required to have prior radiation therapy or at least one systemic therapy. BV was given at 1.8 mg/kg once every 3-week schedule up 16 cycles. A total of 131 patients were enrolled in the study. ORR lasting at least four months was significantly higher in BV group (56.3%) than physician choice’s group (12.5%). The median PFS was 16.7 months with brentuximab vedotin versus 3.5 months with physician’s choice. Peripheral neuropathy developed in 67% of patients in the brentuximab vedotin arm (9%, grade 3) versus 6% in the control arm. Other significant side effects of BV were nausea, diarrhea, fatigue, alopecia, vomiting, and decreased appetite. The FDA approved BV for patients with CD30 (+) MF and pcALCL in 2017 after results from the Alcanza study.
Mogamulizumab
Mogamulizumab is a humanized IgG1 monoclonal antibody, targeting the C-C chemokine receptor 4 (CCR4). The antibody has a defucosylated Fc region, which enhances ADCC by augmenting the binding affinity to Fc receptors, especially FcγRIIIa, on the effector cells (88).
CCR4 is expressed on lymphoma cells in T cell lymphomas including ATLL, PTCL and CTCL (89,90). CCR4 has been shown to be overexpressed on the skin lesions in different stages of MF including skin of patch, plaque, tumor MF and Sezary cells (91). Thus, CCR4 became a new target for the treatment of CTCL.
A multi-institutional phase I-II study was conducted to show the safety and efficacy of mogamulizumab in previously treated CTCL patients (92). Forty-one patients were enrolled in the study. No dose-limiting toxicity was observed. The maximum tolerated dose was not reached in phase 1 after IV infusions of mogamulizumab in escalated doses such as 0.1, 0.3, and 1.0 mg/kg once weekly for four weeks. In the phase 2 part; the patients were treated with the dose of 1 mg/kg. ORR was 37% (47% in patients with SS) in the evaluable 38 patients. A phase II multi-center Japanese study showed similar efficacy in CTCL patients. Thirty-seven patients with PTCL and CTCL received mogamulizumab. ORR was 38% in patients with CTCL (93).
A randomized phase III clinical trial (MAVORIC study) compared the efficacy of mogamulizumab with vorinostat in the patients with previously treated CTCL. The patients were randomly assigned to mogamulizumab (1.0 mg/kg intravenously on a weekly basis for the first 28-day cycle, then on days 1 and 15 of subsequent cycles) or vorinostat (400 mg daily) (94). A total of 372 eligible patients were randomly assigned. Mogamulizumab therapy resulted in significantly superior progression-free survival (PFS) compared with vorinostat therapy (median 7.7 months in the mogamulizumab group vs. 3.1 months in the vorinostat group). The ORR was 28% with mogamulizumab versus 4.8% with vorinostat, which was also statistically significant. The most common serious AEs were pyrexia in eight (4%) patients and cellulitis in five (3%) patients in the mogamulizumab group. Treatment-emergent AEs were more common with mogamulizumab versus vorinostat, including infusion-related reactions (33.2% vs. 0.5%) and skin eruptions due to drug (23.9% vs. 0.5%). The study was concluded that mogamulizumab significantly prolonged PFS compared with vorinostat, and could provide a new, effective treatment for patients with MF and SS (94). The FDA has approved mogamulizumab for relapsed/refractory MF and Sezary disease in August 2018. It was approved in Japan in 2012 for the treatment of adult T-cell leukemia lymphoma (ATLL) and subsequently was also approved for CTCL (CCR4+) in 2014.
The use of mogamulizumab has been associated with increased risk of acute graft versus host disease (GVHD) after allogeneic transplantation in Japanese patients with adult T-cell leukemia lymphoma (95). However, the risk of GVHD in patients with CTCL undergoing allogeneic transplantation remains unknown.
Alemtuzumab
Alemtuzumab is a humanized immunoglobulin G1 antibody against the CD52 molecule. CD52 is expressed on normal B and T lymphocytes as well as on malignant B and T cells in lymphoma patients (96,97). Lundin et al. studied the activity of alemtuzumab in CTCL patients. Twenty-two patients with advanced MF and SS were involved in phase II clinical trial (98). The OR rate was 55%, with 32% of patients in complete remission (CR) and 23% in partial remission (PR). Sézary cells were cleared from the blood in 6 of 7 (86%) patients. Alemtuzumab was also found to have activity in patients with heavily treated CTCL with ORR of 38% (99). French investigators recently reported that alemtuzumab could induce a long-term response especially in patients with SS (100). Twenty-three patients with SS and 16 patients with MF were enrolled in the study. All patients received alemtuzumab 30 mg two to three times per week for a median duration of 12 weeks. ORR was 51% in all patients, 70% in patients with SS, and 25% in patients with MF. Five SS patients and 1 MF patient remained relapse-free for more than two years. The most common side effects are infectious complications including CMV activation because of severe immunosuppression (98,100,101). While not FDA approved for CTCL, and currently not available on the market since its FDA approval for patients with multiple sclerosis in November 2014 under the trade name Lemtrada (Genzyme, Cambridge, MA), alemtuzumab can be obtained for compassionate use from Sanofi for patients with SS. Because of the risk of opportunistic infection, patients receiving alemtuzumab should receive anti-fungal, anti-viral, and PCP prophylaxis.
Other targeted therapy: DD
DD or DAB389IL-2, is a recombinant IL-2-diphtheria toxin fusion protein (102). DD specifically binds to IL-2 receptor, which is expressed on activated T-cells, B-cells, and natural killer (NK) cells. The IL-2 receptor can be divided into three subtypes, such as low, intermediate and high-affinity receptors. DD can bind selectively to the high-affinity IL-2R and then internalized via receptor-mediated endocytosis. Upon acidification of the formed vesicle, the enzymatic portion of the fusion protein moves into the cytosol where it ultimately inhibits protein synthesis and leads to cell death (103).
CTCL cells express IL-2 receptor on their surface in up to 50% of the cases (103). A phase I trial of the first form of DD protein was conducted in the early 90s (104). A phase II study involving 14 patients with CTCL proved a clinical efficacy of DD (105). The expression of the IL-2R may be necessary but is not sufficient to predict response.
A new form of the fusion protein (DAB389IL-2 with higher affinity to IL-2R was developed and investigated in the clinical trials. In phase I/II clinical trial showed that DD has significant activity in CTCL patients with ORR of 34% (106). The pivotal randomized phase III multicenter trial compared two different dose regimens of DD in patients with stage IB to IVA CTCL (107). Patients were randomly assigned to receive either a 9 or 18 µg/kg/day dose for five consecutive days; treatment was repeated every 21 days for up to 8 cycles. Seventy-one patients were enrolled in the clinical trial. ORR was 30% with CR of 10%, and there is no difference between two different dose regimens.
Then, a placebo-controlled phase III clinical trial was conducted to explore the efficacy of DD (108). A total of 144 CTCL patients with CD25 expression were enrolled in the study. ORR was 44% for all participants treated with DD (n=100; 10% CR and 34% PR) compared with 15.9% for placebo-treated patients. PFS was significantly longer (median, >2 years) for both DD doses compared with placebo. Major adverse effects were nausea, pyrexia, fatigue, rash, liver function abnormalities, vision changes, and capillary leak syndrome
DD was also used in combination with bexarotene to enhance the efficacy of the treatment (19). ORR was 67%, and modulation of IL-2R expression was observed with bexarotene combination.
In 1999, DD received accelerated approval from FDA for treatment of CTCL. FDA then approved in 2008 for patients with resistant and recurrent CTCL (full approval). Production of DD (ONTAK) was discontinued in 2014. It is not available in the US at this point.
Systemic chemotherapy
Numerous systemic chemotherapy agents have activity in CTCL, but they do not provide long-term disease control (33). In the study by Hughes and colleagues already cited, the TTNT for single- or multi-agent chemotherapy was only 3.9 months. IFN-α and HDACi provided a better TTNT than chemotherapy (discussed in IFN-α section). Systemic chemotherapy could be used in advanced disease with disseminated and/or bulky nodes, disseminated tumors and visceral involvement (109). A study by the Cutaneous Lymphoma International Consortium (CLIC) analyzed 853 patients with advanced stage MF/SS from 21 centers. Giving chemotherapy as first-line therapy is associated with a higher risk of death, and change of treatment (110). Therefore, chemotherapy should be reserved as an option for the treatment of the patients with advanced disease that have recurrent/relapsed disease after previous therapy(s) (101,109). Systemic chemotherapy could also be considered for patients with aggressive disease with disseminated bulky nodes, disseminated tumors and visceral involvement (109).
Gemcitabine
Gemcitabine (2’,2’-difluorodeoxycytidine) is a pyrimidine antimetabolite results in impaired DNA synthesis and apoptosis of tumor cells. Gemcitabine has been shown activity against CTCL in phase II studies. An Italian group evaluated the efficacy of Gemcitabine as frontline treatment in patients with T cell lymphomas (26 out of 32 were MF) (111). Seven (22%) achieved a CR with ORR of 75%. ORR was similar in MF patients as of 73%. Investigators from MDACC studied the activity of gemcitabine monotherapy in heavily pretreated patients with CTCL (112). Seventeen of 25 patients (68%) responded including 2 CR with tolerable side effects. Pellegrini and colleagues reported that the long-term outcome of the patients with advanced stage CTCL treated with gemcitabine (113). They found the ORR of 48% with CR of 20%. The estimated overall survival was 47% at 15 years, and median DFS reached at 2.9 years. The study confirmed gemcitabine monotherapy as effective and safe in patients with CTCL. Gemcitabine was also combined with bexarotene to enhance the treatment efficacy, but ORR of the combination was not better than previously reported a single agent gemcitabine efficacy in the patients with CTCL (21).
Pegylated liposomal doxorubicin (Doxil)
The pegylated liposomal form of doxorubicin results in reduced toxicity, and a longer half-life (109). It is currently the most commonly used anthracycline in the treatment of CTCL (101). Wollina and colleagues evaluated 10 patients, treated with pegylated liposomal doxorubicin at a dose of 20 mg/m2. ORR was 80% with a high CR rate of 60% (114). Mean DFS was 13.3 months. A multicenter study retrospectively analyzed 34 patients to determine the efficacy of pegylated liposomal doxorubicin (115). Most of the patients received the medication with a dose of 20 mg/m2. ORR was 88.2% with a CR of 44%. Recently, a phase II trial was conducted to find out the efficacy of pegylated liposomal doxorubicin and maintenance bexarotene (Bex) combination. The patients received pegylated liposomal doxorubicin 20 mg/m2 IV, every two weeks for 16 weeks (8 doses) followed by 16 weeks with Bex 300 mg/m2 orally. ORR was 14 out of 34 (41%). Median PFS was five months. Sequential Bex did not increase the response rate or duration (116). We consider that pegylated liposomal doxorubicin continues to play a role in the treatment of recurrent/resistant CTCL.
Pentostatin
Pentostatin is an adenosine deaminase inhibitor selectively toxic to lymphocytes (109). Multiple studies show pentostatin has activity in patients with relapsed/resistant CTCL. Griener et al. treated 18 CTCL patients with pentostatin and found an ORR of 39% (117). Foss et al. reported 94 patients who received pentostatin in 5 phase-II clinical trials with ORR of 40% and CR of 7% (118). The combination with IFN-α resulted in similar ORRs (41% and 51%) (45,46). Moreover, adding IFN could not prolong the duration of the response.
Other chemotherapy medications
Multiple drugs have been reported with activity on CTCL, including bendamustine (119), and clorambucil (120).
Combination chemotherapy
Combination chemotherapy regimens, such as cyclophosphamide-vincristine and prednisone (CVP), cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP), or cyclophosphamide-vincristine-procarbazine and prednisone (COPP) have been previously reported with high response rates in relapsed/resistant CTCL patients (121-123). But, responses do not last. However, Akpek and colleagues treated 15 CTCL patients with EPOCH chemotherapy (124). All patients had advanced refractory disease. EPOCH chemotherapy resulted in an impressive outcome. After a median five cycles of chemotherapy, ORR was 80% with 4 CR (27%), and median PFS was eight months. Fludarabine and cyclophosphamide combination showed ORR of 58% in patients in advanced stage CTCL with time to relapse of ten months (125). The combination chemotherapy especially EPOCH can be considered for advanced stage/refractory CTCL patients.
Specific considerations for SS
SS is clinically characterized by circulating lymphoma T cells, erythroderma and pruritus. Sezary cells commonly express a memory T-cell phenotype such as CD3, CD4, and CD45RO, with an expression of chemokine receptors CCR4, and CCR7 (126). Flow cytometry of peripheral blood often shows the loss of either CD7 or CD26 (127). The aim of systemic therapy is specifically targeting the blood involvement and skin involvement of lymphoma. Systemic therapy plays a central role in the SS [reviewed in (128)]. ECP, bexarotene, IFN-α and low dose PO MTX can be used in the first line therapy of SS either as monotherapy or as a combination. Alemtuzumab, mogamulizumab, HDACi (romidepsin) could be given as a second line therapy. Selected therapies for SS are shown in Table 6.

Full table
Conclusions
Systemic therapy has a significant role in the treatment of CTCL especially advanced stage disease, which has been evolving with the addition of new antibodies such as brentuximab vedotin and mogamulizumab to previously approved medications including retinoids, and HDAC inhibitors. Some of the standard chemotherapy agents like gemcitabine and pegylated liposomal doxorubicin are tolerated well and shown activity in advanced stage CTCL.
All medically fit patients with advanced disease should also be considered for allogeneic transplantation. Numerous new agents; anti-CD3 and anti-CD25 ADCs, immune checkpoint inhibitors, PI-3 Kinase inhibitors and anti-microRNA 155 are being studied by clinical trials. Identifying the patients with high-risk features in the early stage of the disease is a critical step for the development of optimal treatment strategies. Likewise, the best way to sequence or combine therapies in patients with advanced disease remains to be determined.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol 2007;143:854-9. [Crossref] [PubMed]
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007;110:1713-22. [Crossref] [PubMed]
- Horwitz SM, Olsen EA, Duvic M, et al. Review of the treatment of mycosis fungoides and sezary syndrome: a stage-based approach. J Natl Compr Canc Netw 2008;6:436-42. [Crossref] [PubMed]
- Meyer N, Paul C, Misery L. Pruritus in cutaneous T-cell lymphomas: frequent, often severe and difficult to treat. Acta Derm Venereol 2010;90:12-7. [Crossref] [PubMed]
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol 2003;139:857-66. [Crossref] [PubMed]
- Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sezary syndrome. Blood 1996;88:2385-409. [PubMed]
- Virmani P, Hwang SH, Hastings JG, et al. Systemic therapy for cutaneous T-cell lymphoma: who, when, what, and why? Expert Rev Hematol 2017;10:111-21. [Crossref] [PubMed]
- Kim YH, Jensen RA, Watanabe GL, et al. Clinical stage IA (limited patch and plaque) mycosis fungoides. A long-term outcome analysis. Arch Dermatol 1996;132:1309-13. [Crossref] [PubMed]
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol 2010;28:4730-9. [Crossref] [PubMed]
- Talpur R, Singh L, Daulat S, et al. Long-term outcomes of 1,263 patients with mycosis fungoides and Sezary syndrome from 1982 to 2009. Clin Cancer Res 2012;18:5051-60. [Crossref] [PubMed]
- Quaglino P, Pimpinelli N, Berti E, et al. Time course, clinical pathways, and long-term hazards risk trends of disease progression in patients with classic mycosis fungoides: a multicenter, retrospective follow-up study from the Italian Group of Cutaneous Lymphomas. Cancer 2012;118:5830-9. [Crossref] [PubMed]
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol 2013;168:192-200. [Crossref] [PubMed]
- Nieto-Rementería N, Perez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol 2009;160:519-26. [Crossref] [PubMed]
- Duvic M, Hymes K, Heald P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol 2001;19:2456-71. [Crossref] [PubMed]
- Duvic M, Martin AG, Kim Y, et al. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol 2001;137:581-93. [PubMed]
- Talpur R, Ward S, Apisarnthanarax N, et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol 2002;47:672-84. [Crossref] [PubMed]
- Straus DJ, Duvic M, Kuzel T, et al. Results of a phase II trial of oral bexarotene (Targretin) combined with interferon alfa-2b (Intron-A) for patients with cutaneous T-cell lymphoma. Cancer 2007;109:1799-803. [Crossref] [PubMed]
- Kannangara AP, Levitan D, Fleischer AB Jr. Evaluation of the efficacy of the combination of oral bexarotene and methotrexate for the treatment of early stage treatment-refractory cutaneous T-cell lymphoma. J Dermatolog Treat 2009;20:169-76. [Crossref] [PubMed]
- Foss F, Demierre MF, DiVenuti G. A phase-1 trial of bexarotene and denileukin diftitox in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood 2005;106:454-7. [Crossref] [PubMed]
- Dummer R, Beyer M, Hymes K, et al. Vorinostat combined with bexarotene for treatment of cutaneous T-cell lymphoma: in vitro and phase I clinical evidence supporting augmentation of retinoic acid receptor/retinoid X receptor activation by histone deacetylase inhibition. Leuk Lymphoma 2012;53:1501-8. [Crossref] [PubMed]
- Illidge T, Chan C, Counsell N, et al. Phase II study of gemcitabine and bexarotene (GEMBEX) in the treatment of cutaneous T-cell lymphoma. Br J Cancer 2013;109:2566-73. [Crossref] [PubMed]
- Talpur R, Thompson A, Gangar P, et al. Pralatrexate alone or in combination with bexarotene: long-term tolerability in relapsed/refractory mycosis fungoides. Clin Lymphoma Myeloma Leuk 2014;14:297-304. [Crossref] [PubMed]
- Abbott RA, Whittaker SJ, Morris SL, et al. Bexarotene therapy for mycosis fungoides and Sezary syndrome. Br J Dermatol 2009;160:1299-307. [Crossref] [PubMed]
- Spaccarelli N, Rook AH. The Use of Interferons in the Treatment of Cutaneous T-Cell Lymphoma. Dermatol Clin 2015;33:731-45. [Crossref] [PubMed]
- Suchin KR, Cassin M, Gottleib SL, et al. Increased interleukin 5 production in eosinophilic Sezary syndrome: regulation by interferon alfa and interleukin 12. J Am Acad Dermatol 2001;44:28-32. [Crossref] [PubMed]
- Vowels BR, Lessin SR, Cassin M, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol 1994;103:669-73. [Crossref] [PubMed]
- Yoo EK, Cassin M, Lessin SR, et al. Complete molecular remission during biologic response modifier therapy for Sezary syndrome is associated with enhanced helper T type 1 cytokine production and natural killer cell activity. J Am Acad Dermatol 2001;45:208-16. [Crossref] [PubMed]
- Bunn PA Jr, Foon KA, Ihde DC, et al. Recombinant leukocyte A interferon: an active agent in advanced cutaneous T-cell lymphomas. Ann Intern Med 1984;101:484-7. [Crossref] [PubMed]
- Olsen EA, Rosen ST, Vollmer RT, et al. Interferon alfa-2a in the treatment of cutaneous T cell lymphoma. J Am Acad Dermatol 1989;20:395-407. [Crossref] [PubMed]
- Kohn EC, Steis RG, Sausville EA, et al. Phase II trial of intermittent high-dose recombinant interferon alfa-2a in mycosis fungoides and the Sezary syndrome. J Clin Oncol 1990;8:155-60. [Crossref] [PubMed]
- Papa G, Tura S, Mandelli F, et al. Is interferon alpha in cutaneous T-cell lymphoma a treatment of choice? Br J Haematol 1991;79 Suppl 1:48-51. [Crossref] [PubMed]
- Jumbou O, N'Guyen JM, Tessier MH, et al. Long-term follow-up in 51 patients with mycosis fungoides and Sezary syndrome treated by interferon-alfa. Br J Dermatol 1999;140:427-31. [Crossref] [PubMed]
- Hughes CF, Khot A, McCormack C, et al. Lack of durable disease control with chemotherapy for mycosis fungoides and Sezary syndrome: a comparative study of systemic therapy. Blood 2015;125:71-81. [Crossref] [PubMed]
- Stadler R, Otte HG, Luger T, et al. Prospective randomized multicenter clinical trial on the use of interferon -2a plus acitretin versus interferon -2a plus PUVA in patients with cutaneous T-cell lymphoma stages I and II. Blood 1998;92:3578-81. [PubMed]
- Kuzel TM, Roenigk HH Jr, Samuelson E, et al. Effectiveness of interferon alfa-2a combined with phototherapy for mycosis fungoides and the Sezary syndrome. J Clin Oncol 1995;13:257-63. [Crossref] [PubMed]
- Chiarion-Sileni V, Bononi A, Fornasa CV, et al. Phase II trial of interferon-alpha-2a plus psolaren with ultraviolet light A in patients with cutaneous T-cell lymphoma. Cancer 2002;95:569-75. [Crossref] [PubMed]
- Rupoli S, Goteri G, Pulini S, et al. Long-term experience with low-dose interferon-alpha and PUVA in the management of early mycosis fungoides. Eur J Haematol 2005;75:136-45. [Crossref] [PubMed]
- Nikolaou V, Siakantaris MP, Vassilakopoulos TP, et al. PUVA plus interferon alpha2b in the treatment of advanced or refractory to PUVA early stage mycosis fungoides: a case series. J Eur Acad Dermatol Venereol 2011;25:354-7. [Crossref] [PubMed]
- Wozniak MB, Tracey L, Ortiz-Romero PL, et al. Psoralen plus ultraviolet A +/- interferon-alpha treatment resistance in mycosis fungoides: the role of tumour microenvironment, nuclear transcription factor-kappaB and T-cell receptor pathways. Br J Dermatol 2009;160:92-102. [Crossref] [PubMed]
- Dippel E, Schrag H, Goerdt S, et al. Extracorporeal photopheresis and interferon-alpha in advanced cutaneous T-cell lymphoma. Lancet 1997;350:32-3. [Crossref] [PubMed]
- Wollina U, Looks A, Meyer J, et al. Treatment of stage II cutaneous T-cell lymphoma with interferon alfa-2a and extracorporeal photochemotherapy: a prospective controlled trial. J Am Acad Dermatol 2001;44:253-60. [Crossref] [PubMed]
- Raphael BA, Shin DB, Suchin KR, et al. High clinical response rate of Sezary syndrome to immunomodulatory therapies: prognostic markers of response. Arch Dermatol 2011;147:1410-5. [Crossref] [PubMed]
- Roberge D, Muanza T, Blake G, et al. Does adjuvant alpha-interferon improve outcome when combined with total skin irradiation for mycosis fungoides? Br J Dermatol 2007;156:57-61. [Crossref] [PubMed]
- Wagner AE, Wada D, Bowen G, et al. Mycosis fungoides: the addition of concurrent and adjuvant interferon to total skin electron beam therapy. Br J Dermatol 2013;169:715-8. [Crossref] [PubMed]
- Foss FM, Ihde DC, Breneman DL, et al. Phase II study of pentostatin and intermittent high-dose recombinant interferon alfa-2a in advanced mycosis fungoides/Sezary syndrome. J Clin Oncol 1992;10:1907-13. [Crossref] [PubMed]
- Foss FM, Ihde DC, Linnoila IR, et al. Phase II trial of fludarabine phosphate and interferon alfa-2a in advanced mycosis fungoides/Sezary syndrome. J Clin Oncol 1994;12:2051-9. [Crossref] [PubMed]
- Avilés A, Nambo MJ, Neri N, et al. Interferon and low dose methotrexate improve outcome in refractory mycosis fungoides/Sezary syndrome. Cancer Biother Radiopharm 2007;22:836-40. [Crossref] [PubMed]
- Miller CH, Maher SG, Young HA. Clinical Use of Interferon-gamma. Ann N Y Acad Sci 2009;1182:69-79. [Crossref] [PubMed]
- Kurzrock R, Quesada JR, Rosenblum MG, et al. Phase I study of i.v. administered recombinant gamma interferon in cancer patients. Cancer Treat Rep 1986;70:1357-64. [PubMed]
- Kaplan EH, Rosen ST, Norris DB, et al. Phase II study of recombinant human interferon gamma for treatment of cutaneous T-cell lymphoma. J Natl Cancer Inst 1990;82:208-12. [Crossref] [PubMed]
- Dummer R, Hassel JC, Fellenberg F, et al. Adenovirus-mediated intralesional interferon-gamma gene transfer induces tumor regressions in cutaneous lymphomas. Blood 2004;104:1631-8. [Crossref] [PubMed]
- Sugaya M, Tokura Y, Hamada T, et al. Phase II study of i.v. interferon-gamma in Japanese patients with mycosis fungoides. J Dermatol 2014;41:50-6. [Crossref] [PubMed]
- Wood GS, Wu J. Methotrexate and Pralatrexate. Dermatol Clin 2015;33:747-55. [Crossref] [PubMed]
- Zackheim HS, Kashani-Sabet M, Hwang ST. Low-dose methotrexate to treat erythrodermic cutaneous T-cell lymphoma: results in twenty-nine patients. J Am Acad Dermatol 1996;34:626-31. [Crossref] [PubMed]
- Zackheim HS, Kashani-Sabet M, McMillan A. Low-dose methotrexate to treat mycosis fungoides: a retrospective study in 69 patients. J Am Acad Dermatol 2003;49:873-8. [Crossref] [PubMed]
- Vonderheid EC, Sajjadian A, Kadin ME. Methotrexate is effective therapy for lymphomatoid papulosis and other primary cutaneous CD30-positive lymphoproliferative disorders. J Am Acad Dermatol 1996;34:470-81. [Crossref] [PubMed]
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood 2011;118:4024-35. [Crossref] [PubMed]
- Schmid FA, Sirotnak FM, Otter GM, et al. New folate analogs of the 10-deaza-aminopterin series: markedly increased antitumor activity of the 10-ethyl analog compared to the parent compound and methotrexate against some human tumor xenografts in nude mice. Cancer Treat Rep 1985;69:551-3. [PubMed]
- Marchi E, Mangone M, Zullo K, et al. Pralatrexate pharmacology and clinical development. Clin Cancer Res 2013;19:6657-61. [Crossref] [PubMed]
- Rosenstein LJ, Link BK. Optimizing chemotherapeutic strategies for peripheral T-cell lymphomas. Clin Lymphoma Myeloma 2008;8 Suppl 5:S180-6. [Crossref] [PubMed]
- Wang ES, O'Connor O, She Y, et al. Activity of a novel anti-folate (PDX, 10-propargyl 10-deazaaminopterin) against human lymphoma is superior to methotrexate and correlates with tumor RFC-1 gene expression. Leuk Lymphoma 2003;44:1027-35. [Crossref] [PubMed]
- O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol 2011;29:1182-9. [Crossref] [PubMed]
- Horwitz SM, Kim YH, Foss F, et al. Identification of an active, well-tolerated dose of pralatrexate in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood 2012;119:4115-22. [Crossref] [PubMed]
- O'Connor OA, Horwitz S, Hamlin P, et al. Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol 2009;27:4357-64. [Crossref] [PubMed]
- Foss F, Horwitz SM, Coiffier B, et al. Pralatrexate is an effective treatment for relapsed or refractory transformed mycosis fungoides: a subgroup efficacy analysis from the PROPEL study. Clin Lymphoma Myeloma Leuk 2012;12:238-43. [Crossref] [PubMed]
- Duvic M. Histone Deacetylase Inhibitors for Cutaneous T-Cell Lymphoma. Dermatol Clin 2015;33:757-64. [Crossref] [PubMed]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006;6:38-51. [Crossref] [PubMed]
- New M, Olzscha H, La Thangue NB. HDAC inhibitor-based therapies: can we interpret the code? Mol Oncol 2012;6:637-56. [Crossref] [PubMed]
- Moskowitz AJ, Horwitz SM. Targeting histone deacetylases in T-cell lymphoma. Leuk Lymphoma 2017;58:1306-19. [Crossref] [PubMed]
- Butler LM, Agus DB, Scher HI, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res 2000;60:5165-70. [PubMed]
- Zhang C, Richon V, Ni X, et al. Selective induction of apoptosis by histone deacetylase inhibitor SAHA in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. J Invest Dermatol 2005;125:1045-52. [Crossref] [PubMed]
- Garcia-Manero G, Yang H, Bueso-Ramos C, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood 2008;111:1060-6. [Crossref] [PubMed]
- O'Connor OA, Heaney ML, Schwartz L, et al. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol 2006;24:166-73. [Crossref] [PubMed]
- Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 2007;109:31-9. [Crossref] [PubMed]
- Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 2007;25:3109-15. [Crossref] [PubMed]
- Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol 2010;28:4485-91. [Crossref] [PubMed]
- Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol 2009;27:5410-7. [Crossref] [PubMed]
- Duvic M, Bates SE, Piekarz R, et al. Responses to romidepsin in patients with cutaneous T-cell lymphoma and prior treatment with systemic chemotherapy. Leuk Lymphoma 2018;59:880-7. [Crossref] [PubMed]
- Kim M, Thompson LA, Wenger SD, et al. Romidepsin: a histone deacetylase inhibitor for refractory cutaneous T-cell lymphoma. Ann Pharmacother 2012;46:1340-8. [Crossref] [PubMed]
- Sutherland MS, Sanderson RJ, Gordon KA, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem 2006;281:10540-7. [Crossref] [PubMed]
- Schwab U, Stein H, Gerdes J, et al. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature 1982;299:65-7. [Crossref] [PubMed]
- Berger GK, McBride A, Lawson S, et al. Brentuximab vedotin for treatment of non-Hodgkin lymphomas: A systematic review. Crit Rev Oncol Hematol 2017;109:42-50. [Crossref] [PubMed]
- Deng C, Pan B, O'Connor OA. Brentuximab vedotin. Clin Cancer Res 2013;19:22-7. [Crossref] [PubMed]
- Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010;363:1812-21. [Crossref] [PubMed]
- Kim YH, Tavallaee M, Sundram U, et al. Phase II Investigator-Initiated Study of Brentuximab Vedotin in Mycosis Fungoides and Sezary Syndrome With Variable CD30 Expression Level: A Multi-Institution Collaborative Project. J Clin Oncol 2015;33:3750-8. [Crossref] [PubMed]
- Duvic M, Tetzlaff MT, Gangar P, et al. Results of a Phase II Trial of Brentuximab Vedotin for CD30+ Cutaneous T-Cell Lymphoma and Lymphomatoid Papulosis. J Clin Oncol 2015;33:3759-65. [Crossref] [PubMed]
- Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet 2017;390:555-66. [Crossref] [PubMed]
- Ishida T, Iida S, Akatsuka Y, et al. The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-Cell leukemia/lymphoma. Clin Cancer Res 2004;10:7529-39. [Crossref] [PubMed]
- Ishida T, Inagaki H, Utsunomiya A, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res 2004;10:5494-500. [Crossref] [PubMed]
- Duvic M, Evans M, Wang C. Mogamulizumab for the treatment of cutaneous T-cell lymphoma: recent advances and clinical potential. Ther Adv Hematol 2016;7:171-4. [Crossref] [PubMed]
- Sugaya M, Morimura S, Suga H, et al. CCR4 is expressed on infiltrating cells in lesional skin of early mycosis fungoides and atopic dermatitis. J Dermatol 2015;42:613-5. [Crossref] [PubMed]
- Duvic M, Pinter-Brown LC, Foss FM, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood 2015;125:1883-9. [Crossref] [PubMed]
- Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol 2014;32:1157-63. [Crossref] [PubMed]
- Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol 2018;19:1192-204. [Crossref] [PubMed]
- Fuji S, Inoue Y, Utsunomiya A, et al. Pretransplantation Anti-CCR4 Antibody Mogamulizumab Against Adult T-Cell Leukemia/Lymphoma Is Associated With Significantly Increased Risks of Severe and Corticosteroid-Refractory Graft-Versus-Host Disease, Nonrelapse Mortality, and Overall Mortality. J Clin Oncol 2016;34:3426-33. [Crossref] [PubMed]
- Salisbury JR, Rapson NT, Codd JD, et al. Immunohistochemical analysis of CDw52 antigen expression in non-Hodgkin's lymphomas. J Clin Pathol 1994;47:313-7. [Crossref] [PubMed]
- Ginaldi L, De Martinis M, Matutes E, et al. Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leuk Res 1998;22:185-91. [Crossref] [PubMed]
- Lundin J, Hagberg H, Repp R, et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood 2003;101:4267-72. [Crossref] [PubMed]
- Kennedy GA, Seymour JF, Wolf M, et al. Treatment of patients with advanced mycosis fungoides and Sezary syndrome with alemtuzumab. Eur J Haematol 2003;71:250-6. [Crossref] [PubMed]
- de Masson A, Guitera P, Brice P, et al. Long-term efficacy and safety of alemtuzumab in advanced primary cutaneous T-cell lymphomas. Br J Dermatol 2014;170:720-4. [Crossref] [PubMed]
- Photiou L, van der Weyden C, McCormack C, et al. Systemic Treatment Options for Advanced-Stage Mycosis Fungoides and Sezary Syndrome. Curr Oncol Rep 2018;20:32. [Crossref] [PubMed]
- Kaminetzky D, Hymes KB. Denileukin diftitox for the treatment of cutaneous T-cell lymphoma. Biologics 2008;2:717-24. [PubMed]
- Nichols J, Foss F, Kuzel TM, et al. Interleukin-2 fusion protein: an investigational therapy for interleukin-2 receptor expressing malignancies. Eur J Cancer 1997;33 Suppl 1:S34-6. [Crossref] [PubMed]
- LeMaistre CF, Meneghetti C, Rosenblum M, et al. Phase I trial of an interleukin-2 (IL-2) fusion toxin (DAB486IL-2) in hematologic malignancies expressing the IL-2 receptor. Blood 1992;79:2547-54. [PubMed]
- Foss FM, Borkowski TA, Gilliom M, et al. Chimeric fusion protein toxin DAB486IL-2 in advanced mycosis fungoides and the Sezary syndrome: correlation of activity and interleukin-2 receptor expression in a phase II study. Blood 1994;84:1765-74. [PubMed]
- Saleh MN, LeMaistre CF, Kuzel TM, et al. Antitumor activity of DAB389IL-2 fusion toxin in mycosis fungoides. J Am Acad Dermatol 1998;39:63-73. [Crossref] [PubMed]
- Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol 2001;19:376-88. [Crossref] [PubMed]
- Prince HM, Duvic M, Martin A, et al. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. J Clin Oncol 2010;28:1870-7. [Crossref] [PubMed]
- Duvic M. Choosing a systemic treatment for advanced stage cutaneous T-cell lymphoma: mycosis fungoides and Sezary syndrome. Hematology Am Soc Hematol Educ Program 2015;2015:529-44. [Crossref] [PubMed]
- Quaglino P, Maule M, Prince HM, et al. Global patterns of care in advanced stage mycosis fungoides/Sezary syndrome: a multicenter retrospective follow-up study from the Cutaneous Lymphoma International Consortium. Ann Oncol 2017;28:2517-25. [Crossref] [PubMed]
- Marchi E, Alinari L, Tani M, et al. Gemcitabine as frontline treatment for cutaneous T-cell lymphoma: phase II study of 32 patients. Cancer 2005;104:2437-41. [Crossref] [PubMed]
- Duvic M, Talpur R, Wen S, et al. Phase II evaluation of gemcitabine monotherapy for cutaneous T-cell lymphoma. Clin Lymphoma Myeloma 2006;7:51-8. [Crossref] [PubMed]
- Pellegrini C, Stefoni V, Casadei B, et al. Long-term outcome of patients with advanced-stage cutaneous T cell lymphoma treated with gemcitabine. Ann Hematol 2014;93:1853-7. [Crossref] [PubMed]
- Wollina U, Graefe T, Kaatz M. Pegylated doxorubicin for primary cutaneous T cell lymphoma: a report on ten patients with follow-up. Ann N Y Acad Sci 2001;941:214-6. [Crossref] [PubMed]
- Wollina U, Dummer R, Brockmeyer NH, et al. Multicenter study of pegylated liposomal doxorubicin in patients with cutaneous T-cell lymphoma. Cancer 2003;98:993-1001. [Crossref] [PubMed]
- Straus DJ, Duvic M, Horwitz SM, et al. Final results of phase II trial of doxorubicin HCl liposome injection followed by bexarotene in advanced cutaneous T-cell lymphoma. Ann Oncol 2014;25:206-10. [Crossref] [PubMed]
- Greiner D, Olsen EA, Petroni G. Pentostatin (2'-deoxycoformycin) in the treatment of cutaneous T-cell lymphoma. J Am Acad Dermatol 1997;36:950-5. [Crossref] [PubMed]
- Foss FM. Activity of pentostatin (Nipent) in cutaneous T-cell lymphoma: single-agent and combination studies. Semin Oncol 2000;27:58-63. [PubMed]
- Damaj G, Gressin R, Bouabdallah K, et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol 2013;31:104-10. [Crossref] [PubMed]
- Coors EA, von den Driesch P. Treatment of erythrodermic cutaneous T-cell lymphoma with intermittent chlorambucil and fluocortolone therapy. Br J Dermatol 2000;143:127-31. [Crossref] [PubMed]
- Tirelli U, Carbone A, Veronesi A, et al. Combination chemotherapy with cyclophosphamide, vincristine, and prednisone (CVP) in TNM-classified stage IV mycosis fungoides. Cancer Treat Rep 1982;66:167-9. [PubMed]
- Grozea PN, Jones SE, McKelvey EM, et al. Combination chemotherapy for mycosis fungoides: a Southwest Oncology Group study. Cancer Treat Rep 1979;63:647-53. [PubMed]
- Hallahan DE, Griem ML, Griem SF, et al. Combined modality therapy for tumor stage mycosis fungoides: results of a 10-year follow-up. J Clin Oncol 1988;6:1177-83. [Crossref] [PubMed]
- Akpek G, Koh HK, Bogen S, et al. Chemotherapy with etoposide, vincristine, doxorubicin, bolus cyclophosphamide, and oral prednisone in patients with refractory cutaneous T-cell lymphoma. Cancer 1999;86:1368-76. [Crossref] [PubMed]
- Scarisbrick JJ, Child FJ, Clift A, et al. A trial of fludarabine and cyclophosphamide combination chemotherapy in the treatment of advanced refractory primary cutaneous T-cell lymphoma. Br J Dermatol 2001;144:1010-5. [Crossref] [PubMed]
- Campbell JJ, Clark RA, Watanabe R, et al. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood 2010;116:767-71. [Crossref] [PubMed]
- Harmon CB, Witzig TE, Katzmann JA, et al. Detection of circulating T cells with CD4+CD7- immunophenotype in patients with benign and malignant lymphoproliferative dermatoses. J Am Acad Dermatol 1996;35:404-10. [Crossref] [PubMed]
- Olsen EA, Rook AH, Zic J, et al. Sezary syndrome: immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC). J Am Acad Dermatol 2011;64:352-404. [Crossref] [PubMed]
- Richardson SK, Lin JH, Vittorio CC, et al. High clinical response rate with multimodality immunomodulatory therapy for Sezary syndrome. Clin Lymphoma Myeloma 2006;7:226-32. [Crossref] [PubMed]
- Booken N, Weiss C, Utikal J, et al. Combination therapy with extracorporeal photopheresis, interferon-alpha, PUVA and topical corticosteroids in the management of Sezary syndrome. J Dtsch Dermatol Ges 2010;8:428-38. [PubMed]
- Querfeld C, Mehta N, Rosen ST, et al. Alemtuzumab for relapsed and refractory erythrodermic cutaneous T-cell lymphoma: a single institution experience from the Robert H. Lurie Comprehensive Cancer Center. Leuk Lymphoma 2009;50:1969-76. [Crossref] [PubMed]
- Quereux G, Marques S, Nguyen JM, et al. Prospective multicenter study of pegylated liposomal doxorubicin treatment in patients with advanced or refractory mycosis fungoides or Sezary syndrome. Arch Dermatol 2008;144:727-33. [Crossref] [PubMed]


