
Thymidylate synthase gene polymorphism predicts disease free survival in stage II–III rectal adenocarcinoma patients receiving adjuvant 5-FU-based chemotherapy
Introduction
Colorectal cancer (CRC) is the fifth most common cancer with an estimated 376.3 thousand new diagnosed cases in China and approximately 191.0 thousand deaths during 2015 (1).
The 5-fluorouracil (5-FU)-based adjuvant chemotherapy is the standard treatment for operable CRC patients (2). Although adjuvant chemotherapy greatly improves disease-free survival (DFS) and overall survival (OS) in the subpopulation of stage II patients with high risk and stage III patients underwent resection and consequent adjuvant chemotherapy, local recurrence or distant metastasis occurs in about 30–50% patients during the course of the disease (2). Prognostic factor for such patients is a prerequisite for realizing individual therapeutics as to adjuvant chemotherapy. A large number of researches have reported a variety of prognostic factors for DFS and OS in stage II–III CRC such as defective mismatch repair (dMMR) (3), gene expression signature (4), and histological index (5).
Besides, pharmacogenetic polymorphism has been attracting attention during the past decade. Thymidylate synthase (TS) is a key rate-limiting enzyme in folate metabolism, participates in DNA synthesis and also serves as the primary molecular target of fluorouracil. Mechanically, TS catalyses the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) to promote DNA synthesis and repair (6). TS has a 28-bp repeat polymorphism in the 5'-untranslated promoter region (5'-UTR) that has been associated with TS expression (7). Several studies have shown that TS protein expression and its activity are higher with the increase of number of repeats (from double repeate-2R to triple repeate-3R or higher) in vitro and in vivo (8-10). However, there were controversies about the prognostic value of TS 5'-UTR tandem repeat polymorphisms for operable CRC patients receiving 5-FU based adjuvant chemotherapy (11-13).
The present study was undertaken to investigate whether the TS 5'-UTR tandem repeat polymorphisms detected from the peripheral blood mononuclear cells have any prognostic value for DFS and OS in rectal adenocarcinoma patients treated with 5-FU-based adjuvant chemotherapy.
Methods
Patients
This retrospective study included 117 pathologically diagnosed rectal adenocarcinoma patients with stage II–III without distant metastasis and without previous or synchronous second tumor who underwent curative resection at the Department of Surgery of our Institute from 2004 to 2015 and received 5-fluoropyrimidine-based adjuvant chemotherapy.
Among them, 66 patients were additionally treated adjuvant radiotherapy. Any patient who received molecular targeted drugs in the subsequent treatment was excluded.
The median age of whole population was 59 years (range: 21–78 years). Each patient provided their written informed consent and the study was approved by the Ethics Committee of the Institute (Number of ethical approval: No. 2017-41).
Treatment
All patients underwent curative resection reaching R0 resection. 77 patients received Dixon surgery and 59.8% patients of the whole population underwent laparoscopic surgery.
All patients received 5-FU-based adjuvant chemotherapy within one month after operation. Radiotherapy was delivered in a total dose of 45 to 50 Gy (1.8 to 2.0 Gy per fraction, 25 to 28 fractions) using 3D CRT.
A part of patients (n=66) received adjuvant concurrent chemoradiotherapy. For these patients treated with capecitabine single-agent regimen, capecitabine was administrated twice daily reaching 1,600 mg/m2 from the first day to the 14th day in each cycle, 2 cycles during concurrent chemoradiotherapy were completed. If 2 cycles of concurrent chemoradiotherapy cannot be finished due to intolerance, the modification of chemotherapy or radiotherapy dose was permitted. 101 patients received with adjuvant radiotherapy after 4 cycles of FOLFOX4 chemotherapy.
Analysis of polymorphisms
A pretreatment blood sample from each enrolled patient was used for genotyping.
DNA was extracted from 2 mL peripheral limosis vein blood obtained before treatment using a QIAamp kit (Qiagen, Hilden, Germany). PCR amplification of the TS promoter enhance region containing the double and triple tandem repeats was carried out using the following primers: forward 5’ AAAAGGCGCGCGGAAGGGGTCCT 3’ reverse 5’ TCCGAGCCGGCCACAGGCAT 3’PCR reactions were carried out in 15 µL volumes comprising 1 µL of DNA preparation, 0.2 U of TaKaRaTaq HS Polymerase (Takara, Japan),primers at a final concentration of 0.4 µM and 10× reaction mix (Mg2+ Plus) (Takara, Japan) containing nucleotides and buffer. A total of 32 PCR cycles were carried out (94 °C for 40 s, 62 °C for 40 s and 72 °C for 1 min) following hotstart at 94 °C for 5 min. The PCR products were then sequenced with a BigDye Terminator Sequencing kit (Applied Biosystems, Foster City, CA) and ABI 3730XL DNA Analyzer.
Observation and follow-up
The duration of follow-up was measured from the date of starting chemotherapy. Follow-up visits were scheduled every 3 months in the first year, every 6 months in the second and third years, and every year thereafter.
Statistical analyses
The associations between TS 5’-UTR polymorphism and different clinicopathological characteristics were analyzed using the Chi-square or Fisher exact test. The Hardy-Weinberg equilibrium was also evaluated by Fisher exact probability test. Kaplan-Meier curve and log-rank test was used to compare the disease-free survival and overall survival in different clinicopathological characteristics and genotypes of TS 5’-UTR polymorphism. Cox proportional-hazards models were conducted to evaluate prognostic factors for OS. Time-dependent receiver operating characteristic curve (time-dependent ROC) was used to evaluate prognostic accuracies of clinicopathological characteristics and the TS 5’-UTR polymorphism for DFS. P<0.05 was considered statistically significant for the two-sides. Time-dependent ROC and comparison of area under curves was performed using R package “timeROC” (version 0.3). All other statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics and TS 5'-UTR polymorphism
The clinicopathological characteristics of 117 patients and the genotype frequencies of TS polymorphisms were presented in Table 1.
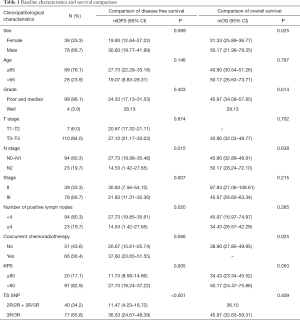
Full table
Among the patients, the distribution of TS 5'-UTR polymorphisms ware 2.6% 2R/2R, 31.6% 2R/3R and 65.8% 3R/3R respectively, which was consistent with Hard-Weinberg equilibrium (χ2=0.345, P=0.558). No significant associations between 5’-TSER polymorphisms and clinicopathological characteristics were observed (Table 2).
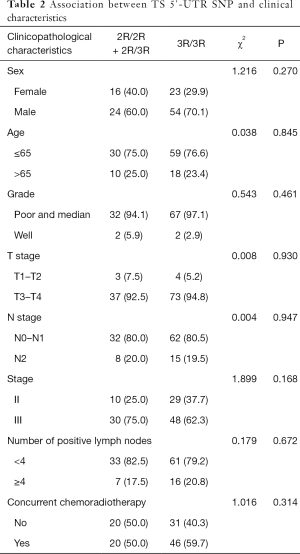
Full table
At the time of the final analysis (July 2016), The median DFS was 24.43 months (95% CI: 20.54–32.32) and the median OS was 45.97 months (95% CI: 35.04–56.90) in this study.
Clinical characteristics and genotypes and survival
As shown in Table 1, later stage, higher N stage, more mesenteric lymph node metastasis, less KPS score and the 5'-UTR polymorphisms (2R/2R+2R/3R) were significantly associated with shorter DFS (Table 1, Figure 1).
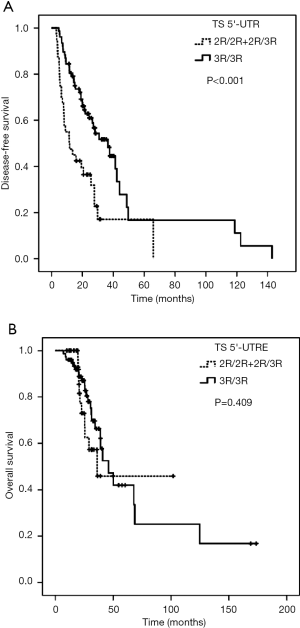
In particular, it was observed that the median DFS of patients harboring 3R homozygous reached 36.53 months, which was significantly longer than that who carrying at least one 5'-TSER 2R allele (Log-Rank P<0.001). With regard to OS, female patients had shorter OS compared with male patients (mOS: 31.33 vs. 50.17 mons, P=0.025). Meanwhile, patients without adjuvant radiotherapy were significantly associated with poor OS (mOS: 38.90 vs. not reached, P=0.025). However, TS 5'-UTR polymorphism had no significant correlation with OS (Table 1, Figure 1).
After adjusted for other baseline clinical characteristics, multivariate Cox regression showed that KPS score and TS 5'-UTR polymorphism were independent prognostic factor for DFS. As regard to TS 5'-UTR polymorphism, the risk of recurrence in patient with 3R/3R homozygous variations was significantly reduced by 56% compared with 2R/2R or 2R/3R patients (HR =0.445, 95% CI: 0.255–0.775, P=0.004). However, KPS score as continuous variable was the only independent prognostic factors for OS (HR =0.910, 95% CI: 0.851–0.972, P=0.005) (Table 3).
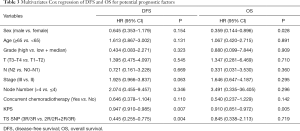
Full table
Subgroup analysis and prognostic accuracy of TS 5'-UTR tandem repeat polymorphisms
In the subset consisted of only female patients, the patients with 3R/3R homozygous variations trend to longer DFS compared with those with 2R/2R or 2R/3R (mDFS: 21.23 vs. 11.37, Log rank P=0.285). In contrast, male patients with 3R/3R homozygous variations exhibited clear longer DFS than those with 2R/2R or 2R/3R (mDFS: 41.33 vs. 11.47, Log rank P=0.001). However, the significance of interaction of genders and TS 5'-UTR tandem repeat polymorphisms was not reached (Pinteraction=0.262). Similar results were obtained regarding to OS. In the female subgroup, patients with 3R/3R had slightly shorter OS compared with those with 2R/2R or 2R/3R (mOS: 31.12 vs. 36.10, Log rank P=0.246). In the male subgroup, patients with 3R/3R trend to be longer OS than those with 2R/2R or 2R/3R (mean OS: 77.64 vs. 63.49, Log rank P=0.186). Again, the significance of interaction of genders and TS 5'-UTR tandem repeat polymorphisms was not reached (Pinteraction=0.102).
Time-dependent ROC analysis was used to evaluate accuracies of baseline clinical characteristics and TS 5'-UTR tandem repeat polymorphisms for 2, 3 and 5 year DFS (Figure 2). The results revealed that TS 5'-UTR tandem repeat polymorphisms had moderate predictive accuracies for 3-year DFS and was parallel with predictive accuracies by stage and number of positive metastatic nodes (Table 4, Figure 2B). However, stage and number of positive metastatic nodes yielded high predictive accuracies for 5-year DFS and significantly outperformed TS 5'-UTR tandem repeat polymorphisms (P=0.007, P=0.0214) (Table 4, Figure 2C).


Full table
Discussion
The present study was showed that for the first time, TS 5'-UTR tandem repeat polymorphisms measured from the PBMC in Chinese rectal adenocarcinoma patients receiving adjuvant 5-FU-based chemotherapy is an independent prognostic factor of DFS.
The distribution of TS 5'-UTR polymorphisms ware 2.6% 2R/2R, 31.6% 2R/3R and 65.8% 3R/3R respectively in our study, which was consistent with those previously reported (14). Moreover, Chen et al. also showed that among Chinese rectal cancer patients, the distribution of TS 5'-UTR polymorphisms ware 4.3% 2R/2R, 28.1% 2R/3R and 67.6% 3R/3R respectively (15).
Overall, the present study showed that patients with TS 5'-UTR 3R/3R had longer DFS compared with patient with TS 5'-UTR2R/2R or 2R/3R.These results was consistent with previous researches results which suggested that germline TS genotypes corresponding to high TS protein expression can predict significantly better DFS and/or OS (8-10). Hitre et al. (13) have reported that the TS 5'-UTR polymorphisms determined from the peripheral blood mononuclear cells have certain prognostic value for disease-free survival (DFS) of colorectal cancer patients treated with adjuvant 5-FU-based chemotherapy. Our cohort is only rectal adenocarcinoma without other histological types in the present study. In our setting, the female patients were found to be associated with inferior OS. This may be attributed to the fact that a part of female patients suffered rectovaginal fistula after concurrent chemoradiotherapy as well as infection which could deteriorate life span. Nevertheless, in subgroup analysis, the gender actually did not modify prognostic effect of TS 5'-UTR tandem repeat polymorphisms.
In contrast, there were many controversial reports on the polymorphism or protein expression of TS as prognostic factors in advanced CRC patients treated with FU chemotherapy. For example, Tan et al. and others demonstrated that TYMS 3R/3R of PBMC might correlate with the poor response (8,12,13,16). Stoehlmacher et al. (17) did not observe any significant difference in the outcome of patients according to TS 5'-UTR genotypes. While Jakobsen et al. (16) and Dotor et al. (18) found better survival outcomes in carriers of TS 5-UTR 3R genotypes than in carriers of the TS 5-UTR 2R/2R genotypes.
The survival of patients with CRC may also be influenced by the folate-deficient state, which can be related to the TS polymorphisms. Spitz et al. (19) showed that, in CRC patients, high folylpolyglutamate synthase (FPGS) gene expression was associated with high TS expression, high total folate levels and better tumor-specific survival. The mucosa samples with high FPGS levels also expressed high TS and elevated total folate levels. While Chen et al. (15) compared with the mean folate level among individuals with the 3R/3R genotype had higher levels of plasma folate. Kim et al. (20) showed that the mean folate level among individuals with the 3R/3R genotype had higher plasma folate levels (>7.72 to ≤11 ng/mL).
Other possible explanations for divergent findings in vivo studies could be contributed to loss of heterozygosity (LOH) in the tumor tissue (16,21) which cause the 2R/loss or the 3R/loss in heterozygous TS 5-UTR 2R/3R risk genotype. Consequently, a proportion of patients expected chemoresistance on the basis of the genomic TS 5-UTR2R/3R status may harbor the favorable 3R/loss genotype in cancer cells (21).
Moreover, inhibition of TS by 5-fluoro-2-deoxyuridine-5-monophosphate does not seem to be the only mechanism of anti-cancer activity of FU. There are other mechanisms including incorporation of 5-fluorouridine-5-triphosphate into RNA and incorporation of 5-fluoro-2-deoxyuridine-5-triphosphate into DNA (22). Finally, Longley et al. (23) demonstrated that certain tumors may still be sensitive to Fas mediated apoptosis by 5-FU despite expressing high levels of TS. This might be a TS independent mechanism.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Each patient provided their written informed consent and the study was approved by the Ethics Committee of the Institute (Number of ethical approval: No. 2017-41).
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Benson AB 3rd, Venook AP, Cederquist L, et al. Colon cancer, Version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:370-98. [Crossref] [PubMed]
- Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011;29:1261-70. [Crossref] [PubMed]
- Song K, Zhao W, Wang W, et al. Individualized predictive signatures for 5-fluorouracil-based chemotherapy in right- and left-sided colon cancer. Cancer Sci 2018;109:1939-48. [Crossref] [PubMed]
- Danielsen HE, Hveem TS, Domingo E, et al. Prognostic markers for colorectal cancer: estimating ploidy and stroma. Ann Oncol 2018;29:616-23. [Crossref] [PubMed]
- Sharp L, Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review. Am J Epidemiol 2004;159:423-43. [Crossref] [PubMed]
- Horie N, Aiba H, Oguro K, et al. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5'-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct 1995;20:191-7. [Crossref] [PubMed]
- Tan BR, Thomas F, Myerson RJ, et al. Thymidylate synthase genotype-directed neoadjuvant chemoradiation for patients with rectal adenocarcinoma. J Clin Oncol 2011;29:875-83. [Crossref] [PubMed]
- Ishida Y, Kawakami K, Tanaka Y, et al. Association of thymidylate synthase gene polymorphism with its mRNA and protein expression and with prognosis in gastric cancer. Anticancer Res 2002;22:2805-9. [PubMed]
- Morganti M, Ciantelli M, Giglioni B, et al. Relationships between promoter polymorphisms in the thymidylate synthase gene and mRNA levels in colorectal cancers. Eur J Cancer 2005;41:2176-83. [Crossref] [PubMed]
- Sulzyc-Bielicka V, Bielicki D, Binczak-Kuleta A, et al. Thymidylate synthase gene polymorphism and survival of colorectal cancer patients receiving adjuvant 5-fluorouracil. Genet Test Mol Biomarkers 2013;17:799-806. [Crossref] [PubMed]
- Edler D, Glimelius B, Hallström M, et al. Thymidylate synthase expression in colorectal cancer: a prognostic and predictive marker of benefit from adjuvant fluorouracil-based chemotherapy. J Clin Oncol 2002;20:1721-8. [Crossref] [PubMed]
- Hitre E, Budai B, Adleff V, et al. Influence of thymidylate synthase gene polymorphisms on the survival of colorectal cancer patients receiving adjuvant 5-fluorouracil. Pharmacogenet Genomics 2005;15:723-30. [Crossref] [PubMed]
- Gao CM, Ding JH, Li SP, et al. Polymorphisms in the thymidylate synthase gene and risk of colorectal cancer. Asian Pac J Cancer Prev 2012;13:4087-91. [Crossref] [PubMed]
- Chen J, Kyte C, Chan W, et al. Polymorphism in the thymidylate synthase promoter enhancer region and risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev 2004;13:2247-50. [PubMed]
- Jakobsen A, Nielsen JN, Gyldenkerne N, et al. Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphism in normal tissue as predictors of fluorouracil sensitivity. J Clin Oncol 2005;23:1365-9. [Crossref] [PubMed]
- Stoehlmacher J, Park DJ, Zhang W, et al. A multivariate analysis of genomic polymorphisms: prediction of clinical outcome to 5-FU/oxaliplatin combination chemotherapy in refractory colorectal cancer. Br J Cancer 2004;91:344-54. [Crossref] [PubMed]
- Dotor E, Cuatrecases M, Martinez-Iniesta M, et al. Tumor thymidylate synthase 1494del6 genotype as a prognostic factor in colorectal cancer patients receiving fluorouracil-based adjuvant treatment. J Clin Oncol 2006;24:1603-11. [Crossref] [PubMed]
- Spitz MR, Wu X, Wang Y, et al. Modulation of nucleotide excision repair capacity by XPD polymorphisms in lung cancer patients. Cancer Res 2001;61:1354-7. [PubMed]
- Kim JW, Jeon YJ, Jang MJ, et al. Association between folate metabolism-related polymorphisms and colorectal cancer risk. Mol Clin Oncol 2015;3:639-48. [Crossref] [PubMed]
- Uchida K, Hayashi K, Kawakami K, et al. Loss of heterozygosity at the thymidylate synthase (TS) locus on chromosome 18 affects tumor response and survival in individuals heterozygous for a 28-bp polymorphism in the TS gene. Clin Cancer Res 2004;10:433-9. [Crossref] [PubMed]
- Hoshino S, Yamashita Y, Maekawa T, et al. Effects on DNA and RNA after the administration of two different schedules of 5-fluorouracil in colorectal cancer patients. Cancer Chemother Pharmacol 2005;56:648-52. [Crossref] [PubMed]
- Longley DB, Allen WL, McDermott U, et al. The roles of thymidylate synthase and p53 in regulating Fas-mediated apoptosis in response to antimetabolites. Clin Cancer Res 2004;10:3562-71. [Crossref] [PubMed]


