
Patient-derived xenografts: a valuable platform for clinical and preclinical research in pancreatic cancer
Introduction
The incidence and mortality of pancreatic cancers (PCs) increased rapidly in worldwide. According to the report in CA, mortality of PC ranks sixth among all malignant tumors in China and mortality due to PC is projected to be second only to Lung cancer by 2030 in the United States (1,2). Although the treatments for PC have made great progress, the 5-year survival rate is still only 5–10% (3). However, most new therapies fail to show significant efficacy in clinical trials due to the high heterogeneity of PC (4-7). Recently, projects such as the Cancer Genome Atlas have highlighted this heterogeneity across tumors previously believed to be of the same subtype (8-10). However, the pre-clinical model based on cancer cell-line cannot represent this heterogeneity. The NCI-60 cancer cell line panels commonly used in basic and pre-clinical research are cultured on the plastic surfaces over many passages and often bear little resemblance to the tumors from which they originated. Cell line-derived xenograft (CDX) models lack the complex component of the human tumor microenvironment and intra-tumoral heterogeneity. To overcome this limitation, xenografts from patient tumor tissues which are known as patient-derived xenografts (PDXs), are better models to preserve the characteristic of the original tumor and provide better tools for translational research and clinical research (11).
PDX is not new. It is first reported in nude mice in 1969, and studies conducted in the 1980s have already observed a similar drug sensitive correlation between PDXs and their donor (11,12). Over the past decade, a number of institutions have established PDX models of PC that collectively represent different subtypes of PC. It is hoped that the advantages of PDXs will promote drug development efficiency and help select drugs based on individual molecular characteristics, paving the way towards precision medicine (13). This review will focus on the application of current PDX models in clinical and preclinical research and how the utility of PDX can be improved in the future.
Generation of PDX
PDX models are generated by implanting fresh patient tumor fragments directly into immunodeficient mice. The process of generating PDX models is extensively described in previous reviews by multiple groups (12-16). Although individual groups have developed specific methodologic approaches, the fundamentals are common. The entire approach is very straightforward. Briefly, pieces of primary or metastatic PC tissue maintained as tissue structures are collected by surgery or biopsy procedures. Ascitic fluid and circulating tumor cells (CTCs) can also be used for implantation (17-20). After division of the tumor into pieces, investigators need implant some pieces embedded in Matrigel, either subcutaneously or orthotopically, into an immunodeficient mouse. The tumors, which are established in immunodeficient mice, are cut into 3–4 mm fragments and reimplanted into new hosts for next passage. The generation of the patient-derived tumor tissue is termed F0, with subsequent generations numbered consecutively (F1, F2, F3 and so on). Different tumor types, implantation sites, and mouse strains can cause differences in PDX tumor formation time. In general, PDX takes 2 to 4 months from inoculation to harvest, and more than 6 months of non-tumor is considered an implantation failure. Drug testing has begun since the third generation (F3) of PDX, and most research groups use F3-F10 for drug treatment (15). Meanwhile, it will be necessary to confirm whether the PDX has diverged from the donor’s tumor in genetics or histology.
Choosing the most appropriate mouse strain to generate PDX model is an important consideration. The PDX model was generated using several different mouse strains with differences in immunosuppression and research benefits. Nude mice are inexpensive and easy to observe the subcutaneous PDX. However, it has intact innate immunity and NK cells like B6 Rag 1 and scid mice, so the tumor formation rate is low. In contrast, NOD scid, NOD.Cg-Prkdcscid IL2rgtm1Wjll/SzJ (NSG) strain and NOD-Cg-Prkdcscid IL2rgtm1Sug/JicTac strain (NOG) have humoral immunity and innate immune deficiency, and PDX is more easily transplanted into these mice. Moreover, NOG and NSG with more severely immunodeficiencies are more suitable for human hematopoietic stem cell transplantation and growth to build humanized mice. This humanized mouse became a humanized PDX model after inoculation of PDX tumors (21-25).
Reported success rates of PDX models estimated by obtaining successful PDX tumors for next passages have ranged between 20% and 80%, depending on the tumor type (16,21,24-36). Even in the same histological origin of same tumor type, the difference is also great. For instance, an estrogen receptor-positive subtype of breast cancer with an engraftment rate under 1% was reported. Conversely, the triple negative subtype, the most aggressive subtype of breast cancer, is much better represented with an engraftment rate of over 35% (11,13). For PC, most of reported engraftment rate is about 60% to 80% (16,32,33,37-44) (Table 1). In general, aggressive and metastatic cancers exhibit higher PDX model transplant rates than less malignant cancers. Compared with tumors with low transplant rate, PDX mice with high transplant rate have lower overall survival rate and increased metastatic events, suggesting that the PDX formation rate can sometimes be used as a predictor of disease prognosis. Meanwhile, successful transplantation will also be affected by the experimental procedure. In PC, implantation with biopsy samples took a reduced engraftment rate (9/24, 37.5%) (44), and only 57/133 (43%) resected tumors with some samples cryopreserved before implantation successfully
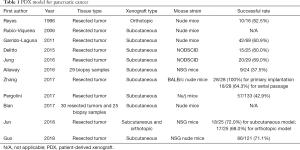
Full table
The rationale behind the development of the PDX model is based on the expectation that these models will preserve the heterogeneity of the tumor and accurately predict the biological behavior of the tumor and its response to treatment. Although cancer cell line, organoid, and CDX models are more suitable for high-throughput drug screening, they have strong selective proliferation and loss tumor heterogeneity (13). Researchers hope that the PDX model can be used as a better preclinical model to facilitated drug development. There is considerable evidence that PDX models retain key features of the patient’s tumor. In PDX tumors, the histological and genomic features of the patient's tumor and the heterogeneity of the tumor cells are highly protected. PDX tumors also contain stroma and immune cells derived from the patient (11,30,32,36,45). Therefore, the PDX model is by far the best preclinical model and represents a highly predictive drug response platform. Researchers not only hope to use this tool to accelerate drug development, but also use this model to guide patients’ treatment to achieve personalized medicine (46).
In sum, these evidences indicate that PDX models can be a stable preclinical research platform that retains the characteristics of original patients’ tumors with regard to histology, genomic and transcriptomic signatures, and drug responsiveness.
Clinical and preclinical applications of PDX models
PDX clinical trial and biomarker screening
There is a large heterogeneity in the molecular characteristics of PC. Therefore, many drugs fail in phase II/III clinical trials. In particular, a series of clinical trials on targeted drugs in PC in recent years have shown that even patients with the same mutation have different response to targeted therapy for this genomic alteration. So, we need a better preclinical platform to further evaluate clinical trial protocols and screen biomarkers. Because the drug response in PDX models is very close to that in clinical practice, it has been widely used in preclinical research. Gao et al. reported the prediction of drug sensitivity in clinical trials using PDX model (47). In drug research, drug susceptibility tests on a certain number of PDX tumor models could infer the general efficiency of the combination in subsequent clinical trials. For example, researchers used 11 PDX models of PC, each of which was divided into 4 groups for drug testing. The results showed that albumin-bound paclitaxel plus gemcitabine had the highest drug response rate (64%) in PDX,compared with albumin-bound paclitaxel alone and gemcitabine alone (48,49). Through the immunohistochemical analysis of PDX tumors, the mechanism of action of albumin-bound paclitaxel combined with gemcitabine was also found, which was to increase the concentration of local gemcitabine by reducing the content of stromal cells in the tumor. Some researchers conducted PDX trial of sirolimus similar to the Phase II clinical trial of on the PDX model, and the results were highly consistent with the drug response rate of the phase II clinical trial (24% vs. 26%) (50). Another drug, AZD0530, were not satisfactory in PDX trial, so the results of the subsequent Phase II clinical study also failed (51). A population-based drug screen has recently been carried out in more than 1,000 PDX models representing a wide range of solid cancers (include PC). Biomarkers of drug sensitivity identified from previous studies were successfully validated in this large panel of PDX models (47). Some novel therapeutic candidates were also detected in PDX trials.
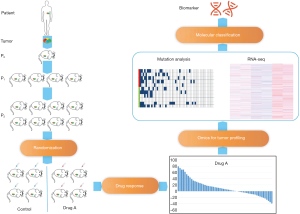
Thus, by using a certain number of unselected PC PDX models, we can perform a statistical, randomized trial (52). Based on the performance of the drug in the PDX trial, we can predict the effectiveness of subsequent clinical trials and improve the design of clinical trials. By the consistency of drug response in PDX model and clinical practice, researchers could screen the corresponding drug biomarkers (Figure 1). The PDX model was divided into sensitive group and insensitive group according to the drug response. The biomarkers were screened by comparing the mutation or expression difference of the corresponding molecules between the two groups. In recent years, studies on drug sensitivity or resistance biomarkers for PC in PDX model have been reported frequently. Golan et al. identified novel biomarkers of PARP inhibitor/cisplatin treatments in PC PDX model (53). Bian et al. reported that PC PDX with an exacerbated expression profile of MYC transcriptional targets (MYC-high) are more sensitive to JQ1 treatment compared to MYC-low PDX. A large-scale PDX trial of PC is needed to validate these biomarkers (43).
PDX are generated in high numbers and extensively characterized at the molecular level and provide a powerful resource for large-scale genotype-response correlations. Thus, PDX model can also be used in basket and umbrella trials. In the era of precision medicine, clinical trials based on tumor sites are too inefficient (54). ‘Basket’ designed clinical trials which are defined as different types of tumors with the same genomic alteration in a clinical trial can significantly improve the efficiency and save research resources. If researchers have a large-scale PDX bank containing multiple tumors, then a basket trial can also be performed on the PDX model. Especially for refractory tumors such as PC, basket trial can provide new treatment options for PC patients with specific targets. If researchers have a PDX panel of PC, an umbrella trial on PDX model can also be performed. Umbrella studies emphasize different treatments for different mutations in patients with same tumor, which is important for PC because the current objective response rate (ORR) of drugs is low. The large-scale PDX bank contains a number of low-frequency driver mutations that allow researchers to conduct appropriate drug test for these PDX. Umbrella study based on the molecular characteristics of each patient's tumor can improve the ORR of PC drugs and reduce the ineffective treatment of patients (Figure 2).
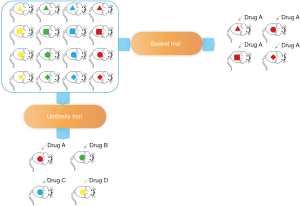
Another significant benefit to performing trials in PDXs (compared with human trials) is that each xenograft can be injected into multiple animals, which allows for direct comparison between drug and control in the same cancer. This direct paired drug trial can directly compare each patient's response to different drugs, which completely excludes the effect of the enrolled population on the drug test results. In theory, this control is more rigorous than human clinical trials and the results are more reliable. In particular, the difference between the two can be seen more intuitively when evaluating the combination and single use.
PDX co-clinical avatar trials
Co-clinical trial refers to simultaneous clinical trials and preclinical PDX trials in which patients and PDX tumors have similar molecular characteristics (55). This idea is based on PDX's accurate drug prediction capability. A phase II or Phase III clinical study often takes several years, and the PDX trial can be completed in a matter of months. By comparing these two trials, we can conduct a series of studies on the mechanism of action of drugs, biomarkers of drug susceptibility and drug resistance (50,56,57). The co-clinical trial was first developed on the genetically engineered mouse models, but was quickly used in the PDX model. Patients enrolled in clinical trials are used to establish the corresponding PDX models. These models receive the same treatment as the patients, and can quickly obtain the drug response, optimize the combination, explore drug resistance mechanisms, and screen for biomarkers (58). Another application of Co-clinical trial is the use of the avatar PDX model in personalized trial. The drug response in the PDX model directly guides the patient’s treatment. Hidalgo et al. conducted clinical trial on metastatic PC used the avatar PDX model to obtain the patient's drug response and optimize therapy (NCT02795650).
PDX model for personalized medicine
Drug selection for patients with PC has relied on evidence from large population-based RCT studies. The first-line drug choices offered by physicians are the best-performing drugs in the population but not necessarily for a single individual patient. Therefore, individualized anti-tumor treatments are developed for each patient to give patients the best way to benefit. This is especially important in PC for the current ORR of first-line drugs is very low and most patients are actually receiving ineffective treatment.
The PDX model relies on its high fidelity for the genetic and pathological features of patients, and is particularly suitable for use in personalized medicine. For example, in patients with PC, researchers can use the surgical sample to establish an avatar PDX model for patients with PC. Once the patient has recurrence and metastasis, the sensitivity of all chemotherapy and targeted drugs for PC can be tested simultaneously, and even drugs for other tumor can be tested in PDX model. According to the test results, we can choose the appropriate treatment. If multiple drugs are sensitive, we can even try combination therapy. Moreover, unlike other individualized drug selection methods, if selected drug exhibits secondary resistance during use, we can re-select the drug through the drug resistance model of PDX mice or build a new model by re-biopsy. This method of personalized treatment still faces many challenges. The first is the tumor formation rate of the PDX model, and not every patient’s tumor can successfully form a PDX. Although the rate of PDX formation in PC is high, only 60–70% of patients can be made their avatar PDX. Then, PDX formation took too much time. At least 3 months of tumor formation time bring a huge clinical challenge. The rapid progression of PC makes it almost impossible for patients to wait for such long-term. Finally, there is also a huge expense. PDX modeling is expensive because it involves costly immunodeficient mice and need multiple passages.
Although these problems are difficult to solve completely, there are some ways to try it in PC. For example, although the tumor formation rate of PC PDX model cannot reach 100%, patients with no PDX formation tend to have a better prognosis, and the possibility of recurrence is lower (32,39,41). So most relapsed PC patients have their avatar PDX model. Secondly, making the PDX model immediately after surgery and carrying out drug test is when the patient is still disease-free, which can directly give the best treatment recommendation when the patient relapses. This method is particularly suitable for PC, because the recurrence rate is extremely high. Finally, screening of multiple drugs can be performed on primary cells and organoid of PC, and then verified by PDX model. This method can greatly save time and money while the accuracy of drug sensitivity is ensured. At present, some researchers and commercial companies are making initial attempts to personalized medicine in PDX model (59-61).
Patient-derived orthotopic xenografts (PDOX)
Although the Subcutaneous PDX model retains some stromal cells, it cannot mimic a tumor microenvironment (62). Because the implantation site not consistent with donor tumors, some pancreatic-specific cells and stroma cannot be retained in the subcutaneous tumor. Of course, there is also a lack of metastatic features of PC. PDOX of PC is a direct injection of PDX tumors into pancreas, primarily the tail of pancreas. The PDOX model has many advantages over the subcutaneous PDX model: (I) a more realistic tumor microenvironment, orthotopic xenograft can better mimic the microenvironment of PC, and the drug sensitivity results are more consistent with patients. Garrido-Laguna et al. changed part of the PDX model to PDOX, the sensitivity of gemcitabine also changed (32); (II) like the PC, the PDOX model will have liver metastases. It can simulate liver metastasis of PC. The difference in drug response between metastases and primary tumors can be compared in the following study. It can also be used to evaluate the effect of adjuvant therapy; (III) for some organ-specific treatments, it must be evaluated by the PDOX model. Cai et al. used an orthotopic pancreatic PDX model to evaluate antitumor effect of apratoxin S10 as Apra S10 tissue distribution indicated its high enrichment in pancreas tissue (63). The large-scale application of PDOX still faces many challenges. The first is that the modeling of the PDOX relies on surgery on mouse, which adds a lot of work. Then in vivo imaging probes is necessary for evaluation, for orthotopic xenografts are deep in the body.
Humanized PDX models
With the rapid development of cancer immunotherapy in recent years, researchers urgently need a PDX model that can be evaluated for immunotherapy (64). However, PDX was modeled on immunodeficient mice and lacked various immune cells (65). Humanized mice are models of human immune system reconstitution in immunodeficient mice (66,67). Combining the humanized mouse model with the PDX model, we can obtain a humanized PDX model (68). Generally, humanized mice can be obtained by injection of peripheral blood mononuclear cells (PBMC) or CD34+ human hematopoietic stem cells (HSCs). However, PBMC may cause severe graft-versus-host reactions (69). The most ideal method is to use the CD34+ HSC from the patient which is the PDX donor, but this is often difficult to achieve. The host selection is generally NOD.Cg-Prkdcscid IL2rgtm1Wjll/SzJ (NSG) strain and NOD-Cg-Prkdcscid IL2rgtm1Sug/JicTac strain (NOG). Although the humanized PDX model is currently the best preclinical model for evaluating immunotherapy, there are still many shortcomings. In particular, the degree of reconstruction of the immune system of humanized mice is different. The difference in the degree of reconstruction of the immune system in mice may bring about different therapeutic effects of immunotherapy. For example, checkpoint therapy often relies on tumor-infiltrating T cells, and we cannot know whether the difference in tumor-infiltrating T cells is caused by humanized immune system or by tumor itself.
CTC-derived PDX models
CTC-derived PDX models are PDX models that directly implant isolated CTCs on immunodeficient mice (17-20). In recent years, many studies have shown that CTCs can detect drug susceptibility in advanced tumors (70-72). The drug susceptibility results of CTC are also consistent with the patient’s primary tumor. Therefore, for patients with advanced cancer who are difficult to perform repeated biopsy, CTC-derived PDX models can be used to assess drug sensitivity of patients. With the development of CTCs separation technology, it has become possible to separate and enrich CTCs. Using CTC-derived PDX models, we can model PDX at multiple time points to assess tumor evolution, tumor resistance mechanisms, and biomarkers. Currently, PC CTC-derived PDX models has not been reported.
Challenges and prospects
Although, the PDX model can well preserve the pathological and molecular characteristics of PC. But there is no denying that the PDX tumor will change with the passage. Recent studies have shown that clonal selection occurs not only in initial engraftment steps but also in propagation steps (73). Moreover, these changes are different from the clonal evolution that occurs when tumors metastasize. Moreover, with the passage of PDX, human stromal and immune cells disappear rapidly, which is particularly important for PC. PC is identified as abundant stroma, and the reduction of human stromal components has an effect on the susceptibility of drugs. Therefore, it is more appropriate to use the early passage of the PDX. The subcutaneous PDX model can have an impact on the response to some drugs, especially anti-angiogenic drugs. Subcutaneous PC PDX has a rich blood supply, which is different from the lack of blood supply for PC. However, the PDOX model for PC is lacking the ability to noninvasively and longitudinally monitor PDX tumor growth kinetics and response to therapies. Small animal imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), and positron-emission tomography (PET) are limited with respect to high-throughput implementation and require costly equipment and infrastructure and a high level of technical expertise.
Organoid model is an in vitro model of 3D culture that retains the patient’s pathological and genetic characteristics. So, it is ideal for high-throughput drug screening. However, the process of 3d culture in vitro inevitably brings artificial selection, resulting in loss of certain tumor heterogeneity in organoid model. Thus, PDX models are still the best model for retaining clinical tumor heterogeneity. Meanwhile, organoid model lacks stromal components, while PC is characterized by abundant stroma. Finally, the organoid model is still an in vitro model that lacks a complex environment in vivo and related metabolic and immune factors. This can lead to inaccuracies in the drug susceptibility results.
With the development of the humanized PDX model, the modeling of humanized mice will become more reliable, and various immune functions in humanized mice need to be evaluated. Currently humanized mice need better. These immune-deficient mice will overcome current shortcomings by genome editing. These modifications will include the replacement or introduction of combinations of human-specific cytokine receptors and adhesion molecules, as well as more comprehensive sets of HLA class I and HLA class II molecules. This will reduce graft-versus-host response and make more complete reconstruction of the human immune system in the mice.
The use of the PDX model in clinical trials relies on a large-scale PDX bank. This is difficult and costly for a research team. Therefore, it is necessary to establish a multi-center collaborative network, which includes not only researchers but also pharmaceutical companies, just like EurOPDX. It may be difficult to set up a PDX consortium of multi-tumor. At least a collaborative network of PC PDX can be established to assemble PDX from various teams to form the PDX encyclopedia of PC, which can greatly accelerate the drug development of PC, explore treatment strategies and bring survival benefits to clinical patients.
Conclusions
The PDX model has been widely used in the past decade. The PC PDX model has been very reliable from modeling, identification and passage. The PDX model of PDX has been used in many applications in PDX trial, drug biomarker screening, PDX co-clinical trial and personalized medicine. At the same time, the new PDOX model, humanized PDX model and CTC-derived PDX model have also developed rapidly. With the cooperation of various research teams of PC PDX, PC PDX will be more widely used in clinical and clinical research.
Acknowledgements
Funding: This study was funded by National Natural Science Foundation of China (81572315), School of Medicine Foundation of Shanghai Jiao Tong University (TM201605), Fostering Fund of Renji Hospital affiliated to Shanghai Jiao Tong University School of Medicine (PYIV-17-001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013;63:318-48. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Electronic address aadhe, Cancer Genome Atlas Research N. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017;32:185-203.e13. [Crossref]
- Oldfield LE, Connor AA, Gallinger S. Molecular Events in the Natural History of Pancreatic Cancer. Trends Cancer 2017;3:336-46. [Crossref] [PubMed]
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52. [Crossref] [PubMed]
- Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun 2015;6:6744. [Crossref] [PubMed]
- Navin N, Krasnitz A, Rodgers L, et al. Inferring tumor progression from genomic heterogeneity. Genome Res 2010;20:68-80. [Crossref] [PubMed]
- McGranahan N, Swanton C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017;168:613-28. [Crossref] [PubMed]
- Hoadley KA, Yau C, Wolf DM, et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 2014;158:929-44. [Crossref] [PubMed]
- Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 2014;4:998-1013. [Crossref] [PubMed]
- Lai Y, Wei X, Lin S, et al. Current status and perspectives of patient-derived xenograft models in cancer research. J Hematol Oncol 2017;10:106. [Crossref] [PubMed]
- Bousquet G, Janin A. Patient-Derived Xenograft: An Adjuvant Technology for the Treatment of Metastatic Disease. Pathobiology 2016;83:170-6. [Crossref] [PubMed]
- Siolas D, Hannon GJ. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res 2013;73:5315-9. [Crossref] [PubMed]
- Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol 2012;9:338-50. [Crossref] [PubMed]
- Reyes G, Villanueva A, Garcia C, et al. Orthotopic xenografts of human pancreatic carcinomas acquire genetic aberrations during dissemination in nude mice. Cancer Res 1996;56:5713-9. [PubMed]
- Williams ES, Rodriguez-Bravo V, Chippada-Venkata U, et al. Generation of Prostate Cancer Patient Derived Xenograft Models from Circulating Tumor Cells. J Vis Exp 2015.53182. [PubMed]
- Toyoshima K, Hayashi A, Kashiwagi M, et al. Analysis of circulating tumor cells derived from advanced gastric cancer. Int J Cancer 2015;137:991-8. [Crossref] [PubMed]
- Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 2014;20:897-903. [Crossref] [PubMed]
- Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 2013;31:539-44. [Crossref] [PubMed]
- Zhang X, Claerhout S, Prat A, et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res 2013;73:4885-97. [Crossref] [PubMed]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1:555-67. [Crossref] [PubMed]
- Vidal A, Munoz C, Guillen MJ, et al. Lurbinectedin (PM01183), a new DNA minor groove binder, inhibits growth of orthotopic primary graft of cisplatin-resistant epithelial ovarian cancer. Clin Cancer Res 2012;18:5399-411. [Crossref] [PubMed]
- Dong X, Guan J, English JC, et al. Patient-derived first generation xenografts of non-small cell lung cancers: promising tools for predicting drug responses for personalized chemotherapy. Clin Cancer Res 2010;16:1442-51. [Crossref] [PubMed]
- Fichtner I, Rolff J, Soong R, et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res 2008;14:6456-68. [Crossref] [PubMed]
- Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 2011;1:508-23. [Crossref] [PubMed]
- Julien S, Merino-Trigo A, Lacroix L, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res 2012;18:5314-28. [Crossref] [PubMed]
- Aytes A, Mollevi DG, Martinez-Iniesta M, et al. Stromal interaction molecule 2 (STIM2) is frequently overexpressed in colorectal tumors and confers a tumor cell growth suppressor phenotype. Mol Carcinog 2012;51:746-53. [Crossref] [PubMed]
- Marangoni E, Vincent-Salomon A, Auger N, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res 2007;13:3989-98. [Crossref] [PubMed]
- DeRose YS, Wang G, Lin YC, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 2011;17:1514-20. [Crossref] [PubMed]
- Li S, Shen D, Shao J, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep 2013;4:1116-30. [Crossref] [PubMed]
- Garrido-Laguna I, Uson M, Rajeshkumar NV, et al. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res 2011;17:5793-800. [Crossref] [PubMed]
- Rubio-Viqueira B, Jimeno A, Cusatis G, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res 2006;12:4652-61. [Crossref] [PubMed]
- Kimple RJ, Harari PM, Torres AD, et al. Development and characterization of HPV-positive and HPV-negative head and neck squamous cell carcinoma tumorgrafts. Clin Cancer Res 2013;19:855-64. [Crossref] [PubMed]
- Keysar SB, Astling DP, Anderson RT, et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol Oncol 2013;7:776-90. [Crossref] [PubMed]
- Némati F, Sastre-Garau X, Laurent C, et al. Establishment and characterization of a panel of human uveal melanoma xenografts derived from primary and/or metastatic tumors. Clin Cancer Res 2010;16:2352-62. [Crossref] [PubMed]
- Jun E, Hong SM, Yoo HJ, et al. Genetic and metabolic comparison of orthotopic and heterotopic patient-derived pancreatic-cancer xenografts to the original patient tumors. Oncotarget 2017;9:7867-81. [Crossref] [PubMed]
- Zhang YJ, Wen CL, Qin YX, et al. Establishment of a human primary pancreatic cancer mouse model to examine and investigate gemcitabine resistance. Oncol Rep 2017;38:3335-46. [PubMed]
- Pergolini I, Morales-Oyarvide V, Mino-Kenudson M, et al. Tumor engraftment in patient-derived xenografts of pancreatic ductal adenocarcinoma is associated with adverse clinicopathological features and poor survival. PLoS One 2017;12:e0182855. [Crossref] [PubMed]
- Delitto D, Pham K, Vlada AC, et al. Patient-derived xenograft models for pancreatic adenocarcinoma demonstrate retention of tumor morphology through incorporation of murine stromal elements. Am J Pathol 2015;185:1297-303. [Crossref] [PubMed]
- Guo S, Gao S, Liu R, et al. Oncological and genetic factors impacting PDX model construction with NSG mice in pancreatic cancer. FASEB J 2019;33:873-84. [Crossref] [PubMed]
- Jung J, Lee CH, Seol HS, et al. Generation and molecular characterization of pancreatic cancer patient-derived xenografts reveals their heterologous nature. Oncotarget 2016;7:62533-46. [Crossref] [PubMed]
- Bian B, Bigonnet M, Gayet O, et al. Gene expression profiling of patient-derived pancreatic cancer xenografts predicts sensitivity to the BET bromodomain inhibitor JQ1: implications for individualized medicine efforts. EMBO Mol Med 2017;9:482-97. [Crossref] [PubMed]
- Allaway RJ, Fischer DA, de Abreu FB, et al. Genomic characterization of patient-derived xenograft models established from fine needle aspirate biopsies of a primary pancreatic ductal adenocarcinoma and from patient-matched metastatic sites. Oncotarget 2016;7:17087-102. [Crossref] [PubMed]
- Sivanand S, Pena-Llopis S, Zhao H, et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci Transl Med 2012;4:137ra75. [Crossref] [PubMed]
- Aparicio S, Hidalgo M, Kung AL. Examining the utility of patient-derived xenograft mouse models. Nat Rev Cancer 2015;15:311-6. [Crossref] [PubMed]
- Gao H, Korn JM, Ferretti S, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med 2015;21:1318-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54. [Crossref] [PubMed]
- Garrido-Laguna I, Tan AC, Uson M, et al. Integrated preclinical and clinical development of mTOR inhibitors in pancreatic cancer. Br J Cancer 2010;103:649-55. [Crossref] [PubMed]
- Rajeshkumar NV, Tan AC, De Oliveira E, et al. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res 2009;15:4138-46. [Crossref] [PubMed]
- Townsend EC, Murakami MA, Christodoulou A, et al. The Public Repository of Xenografts Enables Discovery and Randomized Phase II-like Trials in Mice. Cancer Cell 2016;29:574-86. [Crossref] [PubMed]
- Golan T, Stossel C, Atias D, et al. Recapitulating the clinical scenario of BRCA-associated pancreatic cancer in pre-clinical models. Int J Cancer 2018;143:179-83. [Crossref] [PubMed]
- Janiaud P, Serghiou S, Ioannidis JPA. New clinical trial designs in the era of precision medicine: An overview of definitions, strengths, weaknesses, and current use in oncology. Cancer Treat Rev 2019;73:20-30. [Crossref] [PubMed]
- Nardella C, Lunardi A, Patnaik A, et al. The APL paradigm and the "co-clinical trial" project. Cancer Discov 2011;1:108-16. [Crossref] [PubMed]
- Chen Z, Cheng K, Walton Z, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 2012;483:613-7. [Crossref] [PubMed]
- Jimeno A, Amador ML, Kulesza P, et al. Assessment of celecoxib pharmacodynamics in pancreatic cancer. Mol Cancer Ther 2006;5:3240-7. [Crossref] [PubMed]
- Morelli MP, Calvo E, Ordonez E, et al. Prioritizing phase I treatment options through preclinical testing on personalized tumorgraft. J Clin Oncol 2012;30:e45-8. [Crossref] [PubMed]
- Zhang F, Wang W, Long Y, et al. Characterization of drug responses of mini patient-derived xenografts in mice for predicting cancer patient clinical therapeutic response. Cancer Commun (Lond) 2018;38:60. [Crossref] [PubMed]
- Gilles ME, Hao L, Huang L, et al. Personalized RNA Medicine for Pancreatic Cancer. Clin Cancer Res 2018;24:1734-47. [Crossref] [PubMed]
- Witkiewicz AK, Balaji U, Eslinger C, et al. Integrated Patient-Derived Models Delineate Individualized Therapeutic Vulnerabilities of Pancreatic Cancer. Cell Rep 2016;16:2017-31. [Crossref] [PubMed]
- Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer 2015;15:451-2. [Crossref] [PubMed]
- Cai W, Ratnayake R, Gerber MH, et al. Development of apratoxin S10 (Apra S10) as an anti-pancreatic cancer agent and its preliminary evaluation in an orthotopic patient-derived xenograft (PDX) model. Invest New Drugs 2019;37:364-74. [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Bankert RB, Egilmez NK, Hess SD. Human-SCID mouse chimeric models for the evaluation of anti-cancer therapies. Trends Immunol 2001;22:386-93. [Crossref] [PubMed]
- Holzapfel BM, Wagner F, Thibaudeau L, et al. Concise review: humanized models of tumor immunology in the 21st century: convergence of cancer research and tissue engineering. Stem Cells 2015;33:1696-704. [Crossref] [PubMed]
- Drake AC, Chen Q, Chen J. Engineering humanized mice for improved hematopoietic reconstitution. Cell Mol Immunol 2012;9:215-24. [Crossref] [PubMed]
- Morton JJ, Bird G, Keysar SB, et al. XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene 2016;35:290-300. [Crossref] [PubMed]
- King MA, Covassin L, Brehm MA, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol 2009;157:104-18. [Crossref] [PubMed]
- Yap TA, Lorente D, Omlin A, et al. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res 2014;20:2553-68. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer 2014;14:623-31. [Crossref] [PubMed]
- Ignatiadis M, Lee M, Jeffrey SS. Circulating Tumor Cells and Circulating Tumor DNA: Challenges and Opportunities on the Path to Clinical Utility. Clin Cancer Res 2015;21:4786-800. [Crossref] [PubMed]
- Ben-David U, Ha G, Tseng YY, et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat Genet 2017;49:1567-75. [Crossref] [PubMed]


