
Osimertinib in the treatment of leptomeningeal disease in T790M-negative, epidermal growth factor receptor-mutated non-small cell lung cancer: a case report
Introduction
Leptomeningeal carcinomatosis (LMC) in lung cancer carries a poor prognosis with no standard of care treatment. Its incidence has been increasing due to improved survival of patients from advances in systemic therapy (1). Up to 10% of patients with epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) develop LMC (2). Osimertinib is a third generation EGFR-tyrosine kinase inhibitor (TKI) for the treatment of metastatic EGFR-mutant NSCLC in the setting of acquired T790M mutation (3). It has also been shown to be effective in T790M-negative disease (4). Evidence for the effectiveness of this drug in LMC however is very limited. We report a case of LMC in a patient with T790M-negative EGFR-mutated NSCLC treated with osimertinib, having had previous exposure to erlotinib.
Case presentation
A 74-year-old male with previous history of carcinoembryonic antigen (CEA)-sensitive recurrent NSCLC presented with asymptomatic rise in CEA level. He was diagnosed with stage IB lung adenocarcinoma (AC) 10 years ago treated with right lobectomy. He was then found to have asymptomatic localised paratracheal recurrence 2 years later, discovered incidentally from investigations of an asymptomatic elevation of CEA level. He received concurrent chemoradiation (60 Gy in 30 fractions) with cisplatin/docetaxel as radio-sensitiser. Due to his non-smoking history and Chinese ethnicity, EGFR mutation testing was recommended at the time but declined by the patient due to cost. Positron emission tomography (PET) scan post treatment showed residual soft tissue mass in the right hilar region. Despite unknown EGFR status, he received 5 years of maintenance erlotinib post chemoradiation and was on regular surveillance. He remained well throughout.
He represented twelve months ago again with asymptomatic rise in CEA level (two years after completion of maintenance erlotinib). Computed tomography (CT) chest and PET scan showed stable disease with mild uptake at the right hilar region which was unchanged from previous imaging. Over the next six months, patient remained asymptomatic but CEA continued to rise. Further investigations of repeat imaging including PET scan and CT brain did not reveal a cause. Gastroscopy and colonoscopy showed no evidence of another malignancy. He then complained of new onset headache and intermittent dizziness. General physical and neurological examinations were unremarkable. A magnetic resonance imaging (MRI) of the brain revealed diffuse streaky enhancement within the cerebellar sulci highly suggestive of LMC (Figure 1A). Lumbar punctures showed elevated protein and reduced glucose which would be consistent with LMC but cytology failed to demonstrate any malignant cells. No evidence of disease was found elsewhere on repeat imaging to allow further tissue diagnosis. A presumptive diagnosis of leptomeningeal metastases from recurrent lung AC was made.
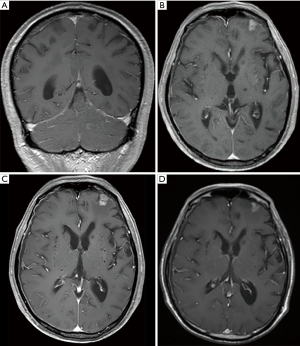
Patient received whole brain radiotherapy 30 Gray in 10 fractions with hippocampal sparing technique. In the interim, clinical deterioration was noted with increased hesitant ambulation, poorer speech and anorexia. His performance status dropped from ECOG 0 to 2. EGFR mutation testing was performed on his lobectomy tissue from 10 years ago which demonstrated TKI-sensitive EGFR G719C and EGFR E709A mutations. Plasma T790M testing was negative. Given previous exposure to erlotinib, he was commenced on osimertinib 80 mg daily, which he tolerated well with no toxicity. His systemic and neurological symptoms improved within 3 weeks of commencing osimertinib. His CEA level fell from 349 ug/L pre-treatment to 71 ug/L at 4 months. Three subsequent MRIs over the next 4 months showed stable disease with unaltered mild patchy enhancement in the cerebellar sulci. The first post treatment MRI did show a small area of irregular enhancing lesion in the left frontal pole measuring <10 mm suspicious for leptomeningeal deposit but the size of this has remained stable over next two scans (Figure 1B,C,D). The patient continues to be well and the treatment remains ongoing after 12 months.
Discussion
While EGFR-TKIs are now widely recognised as first line systemic treatment for metastatic EGFR-mutant NSCLC, evidence for the efficacy of these in the treatment of LMC remains limited. Poor penetration of these drugs through the blood-brain barrier remains a significant issue as over 30% of patients who progress during or after treatment with TKIs have intracranial disease (5). Previous small studies and case series have shown some response using first generation TKIs erlotinib and gefitinib in the treatment of LMC (6-11). It has also been demonstrated that a high dose or pulsatile administration of high dose erlotinib may improve the efficacy of these drugs in the setting of failure of standard daily dosing (12-15).
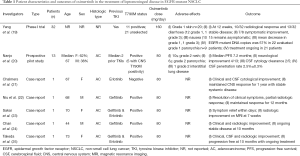
Osimertinib is a third generation TKI developed to target both EGFR-TKI sensitising mutations as well as T790M. It has been shown to be superior over standard platinum-based chemotherapy for the treatment of patients with T790M-positive disease who had progressed after first line TKI (3). It is also superior over first generation TKIs in untreated patients regardless of T790M status (4). In a preclinical study, osimertinib was found to markedly inhibit progression of LMC in in vivo mice model (16). It has also been demonstrated to penetrate the blood brain barrier (BBB) better than gefitinib, afatinib or rociletinib (17). This is supported by sub-analysis of clinical data from the AURA trial, with an overall response rate (ORR) of 70% in patients with brain metastasis treated with osimertinib, compared to 31% in the chemotherapy group (18). The phase 1 BLOOM study is ongoing to assess the activity and safety of osimertinib in the treatment of LMC progressed on prior TKI therapy. Preliminary result on 32 patients at 12 weeks are promising with 23/32 patients achieving a benefit, 10 with radiological response and 13 with stable disease (19). This study included both T790M-positive and unselected patients, it is unknown whether any responders are T790M negative. Several case reports have also demonstrated similar findings (Table 1). However, all except one of these patients had T790M-positive disease.
Full table
The other interesting aspect of this case is the sensitivity of CEA as a tumour marker. Some studies have shown positive results using CEA as a prognostic and/or predictive marker in NSCLC, especially in AC, while others were negative (26). It is also suggested that high CEA level may have a role in predicting development of brain metastasis (27). However, its use is limited to individual patients who have demonstrated CEA sensitive disease as current evidence is inadequate to support its routine use in NSCLC. In our case, CEA was initially requested by the patient’s general practitioner as a non-standard follow-up test but has proven to be a useful marker.
Our case suggests that osimertinib may represent an effective therapeutic option for LMC in EGFR-mutant NSCLC, even in T790M-negative disease. Furthermore, our patient has tolerated treatment extremely well, reflecting clinical trial findings that osimertinib is generally well tolerated with less severe adverse events than first generation TKIs (4). This is especially important to patients with LMC who are likely to have poorer performance status. Nevertheless, our case is limited by the lack of tissue to demonstrate malignant cells and mutation status in the cerebrospinal fluid (CSF). There is a possibility that T790M mutation may be present in the CSF if we were able to obtain any malignant cells.
Conclusions
We herein present a case of leptomeningeal disease in a patient with T790M-negative EGFR-mutant NSCLC achieving significant clinical response using osimertinib. Further prospective studies are needed to evaluate the efficacy and safety of osimertinib in this setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying image.
References
- Lee SJ, Lee JI, Nam DH, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol 2013;8:185-91. [Crossref] [PubMed]
- Kuiper JL, Hendriks LE, van der Wekken AJ, et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer 2015;89:255-61. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M positive lung cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefinitib or erlotinib. Clin Cancer Res 2010;16:5873-82. [Crossref] [PubMed]
- Masuda T, Hattori N, Hamada A, et al. Erlotinib efficacy and cerebrospinal fluid concentration in patients with lung adenocarcinoma developing leptomeningeal metastases during gefitinib therapy. Cancer Chemother Pharmacol 2011;67:1465-9. [Crossref] [PubMed]
- Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol 2009;4:1415-9. [Crossref] [PubMed]
- Yang H, Yang X, Zhang Y, et al. Erlotinib in combination with pemetrexed/cisplatin for leptomeningeal metastases and cerebrospinal fluid drug concentrations in lung adenocarcinoma patients after gefitinib failure. Target Oncol 2015;10:135-40. [Crossref] [PubMed]
- Lee E, Keam B, Kim DW, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small cell lung cancer. J Thorac Oncol 2013;8:1069-74. [Crossref] [PubMed]
- Umemura S, Tsubouchi K, Yoshioka H, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer 2012;77:134-9. [Crossref] [PubMed]
- Park JH, Kim YJ, Lee JO, et al. Clinical outcomes of leptomeaningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer 2012;76:387-92. [Crossref] [PubMed]
- Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 2011;13:1364-9. [Crossref] [PubMed]
- Togashi Y, Masago K, Fukudo M, et al. Efficacy of increased-dose erlotinib for central nervous system metastases in non-small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Chemother Pharmacol 2011;68:1089-92. [Crossref] [PubMed]
- Milton DT, Azzoli GC, Heelan RT, et al. A phase I/II study of weekly high-dose erlotinib in previously treated patients with nonsmall cell lung cancer. Cancer 2006;107:1034-41. [Crossref] [PubMed]
- Kawamura T, Hata A, Takeshita J, et al. High-dose erlotinib for refractory leptomeningeal metastases after failure of standard-dose EGFR-TKIs. Cancer Chemother Pharmacol 2015;75:1261-6. [Crossref] [PubMed]
- Nanjo S, Ebi H, Arai S, et al. High efficacy of third generation EGFR inhibitor AZD9291 in a leptomeningeal carcinomatosis model with EGFR-mutant lung cancer cells. Oncotarget 2016;7:3847-56. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Mok T, Ahn M, Han J, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: data from a randomised phase III trial (AURA3). J Clin Oncol 2017;35:abstr 9005.
- Yang JC, Cho BC, Kim D, et al. Osimertinib for patients with leptomeningeal metastases from EGFR-mutant non-small cell lung cancer: updated results from the BLOOM study. J Clin Oncol 2017;35:abstr 2020.
- Nanjo S, Hata A, Okuda C, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer 2018;118:32-7. [Crossref] [PubMed]
- Chalmers A, Jensen L, Akerley W. Durable response to osimertinib in EGFR mutated T790M wildtype non-small cell lung cancer with leptomeningeal metastases: A case report. Lung cancer 2017;114:68-9. [Crossref] [PubMed]
- Niu H, Zhou J, Maan H, et al. Treatment of leptomeningeal metastases in a patient with non-small cell lung cancer harbouring EGFR T790M mutation. Case Rep Oncol 2017;10:840-5. [Crossref] [PubMed]
- Sakai H, Hayashi H, Iwasa T, et al. Sucessful Osimertinib treatment for leptomeningeal carcinomatosis from lung adenocarcinoma with T790M mutation of EGFR. ESMO Open 2017;2:e000104. [Crossref] [PubMed]
- Chan OS, Leung WK, Yeung RM. Sustained response to standard dose osimertinib in a patient with plasma T790M-positive leptomeningeal metastases from primary lung adenocarcinoma. Asia Pac J Clin Oncol 2017;13:428-30. [Crossref] [PubMed]
- Takeda T, Itano H, Takeuchi M, et al. Osimertinib administration via nasogastric tube in an EGFR-T790M-positive patient with leptomeningeal metastases. Respirol Case Rep 2017;5:e00241. [Crossref] [PubMed]
- Grunnet M, Sorensen JB. Carcinoembryonic antigen as tumour marker in lung cancer. Lung Cancer 76:138-43. [Crossref] [PubMed]
- Arrieta O, Saaveda-Perez D, Kuri R, et al. Brain metastasis development and poor survival associated with CEA level in advanced non-small cell lung cancer: a prospective study. BMC Cancer 2009;9:119. [Crossref] [PubMed]


