Systemic therapy for hepatocellular carcinoma
Introduction
Historically, several therapeutic strategies for the treatment of advanced hepatocellular carcinoma (HCC) have been studied; however, no approach has resulted in an improvement in patient outcomes (1). In the last decade, intensive investigation into the molecular pathogenesis of liver cancer has led to new mechanistic insight, particularly regarding the angiogenic dependence of HCC (2). This has resulted in the successful clinical development of the sorafenib, a multi-kinase inhibitor (3). The success of sorafenib has galvanized the global medical research community, and currently, there are approximately 60 small molecule targeted therapeutics in various stages of clinical development, and over 200 ongoing or completed advanced HCC specific clinical trials worldwide (www.clinicaltrials.gov). Despite these advancements, several critical questions and challenges remain for HCC treatment and drug development. In this manuscript, we will conduct a brief review of the molecular pathogenesis of HCC followed by a discussion of development of anti-angiogenic therapy in this disease. Remaining clinical and translational research questions as well as the challenges of clinical trial design in context of HCC will also be highlighted herein.
Molecular and cellular biology of hepatocellular carcinoma
Hepatocarcinogenesis is a complex, multistep process whereby recurrent hepatic injury results in the accumulation of aberrant genomic, chromosomal, and epigenetic events (4). Such events define the malignant phenotype; activate numerous developmental pathways and signal transduction cascades; disrupt cell-cycle checkpoints and normal apoptotic pathways; and lead to uncontrolled cellular proliferation, growth, survival, and angiogenesis (5).
The WNT/β-catenin pathway, a tightly regulated signaling cascade in normal embryogenesis and hepatocyte differentiation, is heavily dysregulated in HCC (Figure 1). Activating somatic mutations within in the gene encoding β-catenin, CTNNB1 (~30%), or in mutually exclusive inactivating mutations in AXIN1 (~15%) or APC (~2%) have been observed by numerous investigators (6-11). High level chromosomal imbalances also occur on several loci that contain genes known to modulate WNT signaling (i.e., FZD3, WISP1, SIAH-1 and AXIN2) (12). Furthermore, overexpression of FZD7, a component of Frizzled (i.e., the WNT receptor), is observed in up 90% of HCC human tumors (13). The functional consequences of global changes in this pathway as well as the individual contributions of each alteration to tumorigenicity require more detailed characterization. However, it is clear that a large subset (up to 50%) of HCC is characterized by functional WNT pathway activation, and that such aberrant signaling, in part, drives HCC proliferation and growth (14,15). Other developmental pathways are implicated in hepatocarcinogenesis and these include the hedgehog (16), notch (17), and the c-MET proto-oncogene/hepatocyte growth factor receptor (HGF) pathways (18,19).
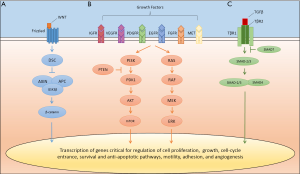
Mitogen-activated signaling cascades are also critical in HCC biology; however, unlike other malignancies, driver mutations in these pathways do not occur at a high frequency (9-11). The phosphatidylinositide 3-kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR) (14,20), and classic mitogen-activated protein kinase (MAPK) (21-23) pathways are activated in HCC (Figure 1). Blockade of these individual signaling cascades suppresses tumor growth in vitro and in vivo (24). Importantly, overproduction of mitogens [i.e., vascular endothelial growth factor (VEGF), and platelet derived growth factor (PDGF)] by the tumor and the surrounding cirrhotic microenvironment serves to sustain the neoplastic clone, drive downstream signaling cascades, and stimulate neo-angiogenesis (2). Over-expression and/or activation of the receptor tyrosine kinases linked to theses oncogenic pathways, including the epidermal growth factor receptor (EGFR) (18), VEGFR-1/-2/-3 (25-27), PDGFR (19), insulin-like growth factor receptor (IGFR) (28), and fibroblast growth factor receptor (FGFR) (29) are frequent in HCC. Finally, impairment of negative regulators of growth factor-dependent signaling, such as decreased PTEN activity in the case of PI3K-AKT-mTOR pathway, serves to further deregulate normal signals for growth and cell survival (30).
Evasion of normal apoptotic mechanisms and cell-cycle checkpoints by HCC also promote cancer formation and progression. Transforming growth factor (TGF)-β, via the SMAD proteins and other downstream effectors, exhibits potent anti-proliferative properties in normal hepatocytes (Figure 1) (31). Alterations in this pathway, particularly loss of SMAD4, can result in escape of the growth inhibitory properties of TGF-β (32). In this setting, TGF-β paradoxically promotes growth, invasion, and angiogenesis, and induces epithelial-mesenchymal transition (31). TP53, a tumor suppressor gene and cell-cycle checkpoint, is inactivated by somatic mutation in up to 50% of HCC (9,10). Further, impairment of RB1/p16 function, which limits cell replication in the setting of DNA damage, is suppressed by promotor hypermethylation and other mechanisms in a majority of tested tumors (33). Finally, alterations in epigenetic modifiers (ARID1/2, MLL, MLL3 and others) (10,11) and mutations within non-coding regulator promoters (TERT) (10,34) are common and the implications of these changes are only now being explored.
Moving forward continued molecular characterization of HCC will likely clarify the consequences of the above alterations and give insight into new therapeutic targets and novel combination strategies. Although targeting WNT appears to be priority in HCC, “drugging” this pathway has been difficult and we are only now seeing these compounds entering phase I clinical trials. Agents predicted to impair HCC growth, specifically by blocking VEGF signaling and other related mitogen-activated signal transduction cascades, have been extensively studied. The ensuing discussion will focus on the successes, failures, and ongoing studies in this area.
Inhibition of angiogensis
Sorafenib
Sorafenib is a small molecule that targets tumoral angiogenesis and neoplastic proliferation leading to tumor-cell apoptosis in preclinical models (35). Its anti-angiogenic effects are thought to be mediated by blockade of VEGFR-2/-3, PDGFR-β, and other receptor tyrosine kinases. The compound also appears to inhibit the RAF kinases, critical components of the MAPK pathway, in both biochemical and cellular experimental systems. Given that the molecular pathogenesis of HCC is dependent upon both exuberant angiogenesis mediated, in part, by VEGF (2), and aberrant MAPK signaling (21-23), strong preclinical rationale exists for sorafenib as a therapy in HCC. Several clinical trials established the utility of sorafenib in this disease, and as such, the European Commission and the United States Food and Drug Administration licensed it for the treatment of advanced HCC in 2007 (3,36-39). In the subsequent year, the State Food and Drug Administration of China and other international agencies approved sorafenib for the same indication.
The clinical efficacy of sorafenib in HCC was firmly established by a multicenter phase II study (3). One-hundred and thirty-seven patients with systemic treatment-naïve, inoperable HCC and varying hepatic reserve (72% Child-Pugh A, 28% Child-Pugh B) received the agent. The primary objective of the study was to determine the objective response rate to sorafenib, and the predefined boundary to establish cytotoxic efficacy was set at a 7% confirmed response rate. Although only 2.2% of the study population achieved a confirmed objective response by WHO criteria, 42% percent of the study population had extended disease control. The median overall survival was 9.2 months, which was encouraging when compared to historical controls. A second study composed exclusively of an Asian population obtained similar favorable results (37).
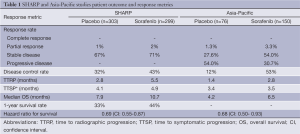
Subsequently, two pivotal, multicenter, double-blind, placebo-controlled, randomized phase III studies of sorafenib versus best supportive care in patients with advanced HCC demonstrated a statistically significant improvement in overall survival in favor of sorafenib (Table 1) (38,39). The SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol) trial enrolled 602 patients with advanced HCC who had not received prior systemic therapy (39). The majority of the study population, which was recruited predominately from Europe and Australasia, had HCC with macroscopic vascular invasion, extrahepatic spread or both. Preserved liver function was a strict inclusion criterion of the study, and in fact, only 3.3% of participants had Child Pugh class B hepatic function. HCC etiologic factors were well distributed amongst participants with roughly 28%, 26%, and 18% of cases related to HCV, alcohol, and HBV, respectively. Patients were randomly assigned to receive sorafenib at 400 mg orally twice a day (n=299) or best supportive care (n=303). The co-primary endpoints of the study were overall survival and time to symptomatic progression. Sorafenib rarely resulted in tumor shrinkage; however, the agent was associated with an absolute increase in the disease control rate of 11% when compared with placebo. This cytostatic effect translated to a statistically significant longer time to radiographic progression and an absolute 11% increase in the 1-year survival rate. Median overall survival was 10.7 months in the sorafenib arm versus 7.9 months in the cohort receiving best supportive care (HR=0.69, 95% CI: 0.55-0.87). Predefined subset analysis indicated that the survival benefit of sorafenib was independent of performance status and disease burden.
Full table
Designed in parallel with SHARP, the Asia-Pacific study assessed the efficacy and tolerability of sorafenib in comparison with best supportive care in the patients with advanced HCC geographically localized to China, South Korean, and Taiwan (38). The study was therefore well positioned to assess the potential impact of known regional differences in HCC etiologic factors on responsiveness to treatment. By providing a closer representation of the worldwide HCC patient population, the Asia-Pacific study also minimizes theoretical confounding factors (e.g., environmental aflatoxin exposure, socioeconomic variables, etc.) that might be unique to Asia and not adequately represented by the SHARP study population. As expected and in contrast to SHARP, the Asia-Pacific study was enriched with patients with HBV-related HCC (73% of the total study population), and in general, was compromised of a greater proportion of patients with poorer ECOG performance status and greater disease burden. Despite these differences, the trial confirmed that sorafenib, when compared to best supportive care, was tolerable and led to a statistically significant improvement in disease control, time to radiographic progression, and overall survival.
It is important to note that the magnitude of the overall survival benefit on the Asia-Pacific study was not as substantial as observed on the SHARP study—the median overall survival was only 6.5 and 4.2 months for patients receiving sorafenib and placebo, respectively. The inclusion of patients who were more ill prior to beginning therapy than those patients on the SHARP study might, partly or even fully, explain this slight survival difference. Another postulate is that the observed differential outcomes on the two trials were due to differing treatment patterns between Asia and Western countries. Aggressive local regional therapies might be more common in Asia, thus leading to the selection of patients on the Asia-Pacific study who are presenting later in the course of their disease. The inclusion criteria for the Asia-Pacific study; however, do not necessarily support this assertion. Alternatively and provocatively, specific viral etiologic factor might affect prognosis and influence the responsiveness of liver cancer to sorafenib.
In an unplanned subset analysis of the SHARP study, patients with HBV-related HCC (n=60) who were treated with sorafenib had a modest prolongation in median overall survival over placebo (9.7 vs. 6.1 months) but similar disease control rates (34.4% vs. 32.1%) and near equivalent time to progression (2.7 vs. 4.2 months) (40). In contrast, HCV-related HCC patients (n=167) treated with sorafenib appeared to derive much greater clinical benefit, with substantial improvements over placebo in overall survival (14.0 vs. 7.4 months), disease control rates (44.2% vs. 29.6%), and time to progression (7.6 vs. 2.8 months). Retrospective analysis of initial phase II study of sorafenib observed similar etiologic-dependent trends in survival (41). Patients who were infected with HCV lived longer (n=13, 12.4 months) than did patients infected with HBV (n=33, 7.3 months, P=0.29). Finally, the recently reported phase III study of first-line sunitinib indicates that there may in fact be differential outcomes relative to disease cause and ethnic origin, with median overall survival for HCV-associated HCC ranging from 18.3 months for patients with living outside of Asia to 7.9 months for patients living in Asia (42).
A caveat to drawing a firm conclusion on the matter of variable sensitivity to sorafenib is that sample size is small and ad hoc subgroup analyses are notoriously subject to confounding secondary to population imbalance. Certainly if differentially, antitumor activity exists, etiologic-dependent genomic differences in HCC might explain improved outcomes to sorafenib in patients with HCV-related HCC. CTNNB1 mutations are more commonly observed in HCV-related but not in HBV-related HCC and are associated with a specific WNT gene expression profile (9,14,15). Sorafenib can modulate this gene signature, interfere with WNT signaling output, and lead to HCC growth suppression in preclinical models (15). Etiologic-dependent differences in outcome might also be explained by HCV core protein-induced upregulation of the sorafenib target CRAF, among other kinases (43). Finally, in vitro data suggest that sorafenib can directly inhibit HCV viral replication, though the clinical importance of this observation is debatable (44). Although more exploration is certainly required, it should be emphasized that the utility of sorafenib is not undercut by this observation and it remains an effective and life prolonging therapy for HCC, irrespective of etiologic factor.
Sorafenib combination strategies
In the attempt to improve upon the modest results observed with sorafenib, investigators have proposed combination strategies with cytotoxic chemotherapy and novel biologic agents. Prior to the approval of sorafenib, doxorubicin was evaluated as monotherapy or in combination with sorafenib in a randomized, double blind, phase II study (45). The trial enrolled 96 patients with treatment-naïve advanced HCC and Child-Pugh A liver function. The primary endpoint of the study was time to progression. Importantly, both time to progression, as determined by independent review, and progression-free survival were increased by approximately 4 months, and the median overall survival doubled in favor of combined therapy (13.7 vs. 6.5 months, P=0.006). Cardiac toxicity was notable, with a higher proportion of patients on the combination experiencing left ventricular systolic dysfunction (19% vs. 2%). Although the majority of such cases were asymptomatic, the median cumulative doxorubicin dose was limited to 165 mg/m2.
The dramatic increase in survival over placebo was striking; however, the lack of sorafenib as a comparator arm limits the interpretation of the trial. Doxorubicin may contribute little to outcome. The observed benefit in the doxorubicin-sorafenib group may be due to the effects of sorafenib alone. Alternatively, the combination may be synergistic. Inhibition of the MAPK pathway by sorafenib may restore chemosensitivity by enhancing pro-apoptotic pathways and dampening multi-drug resistance (MDR) pathways. Anthracycline-induced cytotoxicity is mediated by the pro-apoptotic kinase ASK1 (46). Growth factor-induced MAPK activation, via FGF, has been shown to abrogate ASK1 activity. Blockade of the RAF kinases by sorafenib might therefore augment the antitumor activity of doxorubicin. Furthermore, MAPK activation leads to the induction of MDR-1 pump (47). Sorafenib decreases ATP-binding cassette/MDR protein gene expression thereby restoring HCC sensitivity to doxorubicin in vitro (48). A randomized phase III study of sorafenib versus sorafenib and doxorubicin in the first-line setting (www.clinicaltrials.gov NCT01015833) and a phase II study of the regimen in second-line setting after sorafenib failure (www.clinicaltrials.gov NCT01840592) are currently underway.
Gemcitabine and oxaliplatin (GEMOX) therapy has established efficacy in HCC (49), and there is reason to believe that addition of sorafenib to gemcitabine might offer synergistic anti-tumor effects (48). GEMOX-sorafenib versus sorafenib was recently tested in a randomized phase II study (GONEXT) (50). The trial enrolled 95 patients with advanced HCC (CLIP 52% 2/3), excellent performance status (69% WHO PS 0), and Child-Pugh A liver function. The primary endpoint was 4-month progression—free survival of greater than or equal to 50%. The combination of GEMOX plus sorafenib resulted in a 4-month PFS rate of 61% compared to 54% in sorafenib monotherapy group. The combination was feasible and efficacy data were encouraging (ORR 16%, DCR 77%), though grade 3/4 neutropenia, fatigue, thrombocytopenia, diarrhea, and sensory neuropathy were common. More data will be required to define the role of this sorafenib combination strategy in HCC. In addition, several other trials are evaluating sorafenib in combination with other forms of cytotoxic chemotherapy.
In addition to its application with anti-angiogenic agents such as bevacizumab, sorafenib is being combined with antisense technologies; receptors tyrosine kinase inhibitors and monoclonal antibodies blocking EGFR, c-MET, FGFR and IGFR; multiple small molecule inhibitors of the MAPK and PI3K-AKT-mTOR pathways; histone deacetylase inhibitors; and novel immune-based therapies. The majority of these biologic combinations are still in early drug development and it is premature to comment on how they might improve upon sorafenib, though emerging data are promising and there remains enthusiasm for drug development in this area.
Erlotinib, an EGFR tyrosine kinase inhibitor, and sorafenib are the first novel pairing to reach later stages of clinical development. Although there is a theoretical benefit to blocking both EGFR and VEGFR in HCC, the addition of erlotinib to sorafenib did not produce additive or synergistic effects in vitro or in vivo (51). A phase I study that evaluated sorafenib and erlotinib in 17 patients with various solid tumors, included a single case of HCC (52). This patient received the recommended phase II dose and had a best overall response of stable disease with ~5% tumor growth on study. In an extension cohort of this trial, an additional evaluable HCC patient progressed after 75 days of combination therapy (53). The SEARCH trial confirmed that the addition of erlotinib to sorafenib provided no benefit in HCC (54). In this randomized, placebo controlled, double blind, phase III study the combination of sorafenib and erlotinib were compared to sorafenib alone in the first-line setting in 720 patients with advanced HCC. There was no statistically significant difference between study arms with regard to the primary endpoint of overall survival (combination 9.5 months, sorafenib 8.5 months, HR=0.93, 95% CI: 0.78-1.11).
Multi-targeted receptor tyrosine kinase inhibitors
Several small molecule, orally available, receptor tyrosine kinase inhibitors with the ability to inhibit VEGFR, and other kinases, have undergone extensive evaluation or are being tested in clinical trials of varying stages for the treatment of advanced HCC. These agents include sunitinib, axitinib, regorafenib, brivanib, linifanib, vandetanib, cediranib, pazopanib, TSU-68, vatalanib, and lenvantinib. Thus far, emerging results have been disappointing with the major phase III studies of anti-angiogenic therapy failing to improve upon sorafenib in the first-line setting, and no clear benefit over best supportive care of additional anti-angiogenic monotherapy in the second-line setting.
Sunitinib inhibits VEGFR-1/-2 with greater potency than sorafenib (55). Additionally, the agent targets PDGFR-α/β, c-KIT, FLT3, RET, and other kinases. Three separate phase II studies of sunitinib evaluated three different dosing schedules of the agent as a treatment for advanced HCC (56-58). A subsequent randomized phase III study of sunitinib, dosed continuously, versus sorafenib in patients with advanced HCC and Child Pugh Class A liver function was initiated and rapidly enrolled 1,073 patients (42). The study, powered to test the dual hypotheses of non-inferiority and superiority with regard to overall survival, was halted by an independent data monitoring committee due to futility and safety concerns. Median overall survival for the sunitinib cohort was 8.1 months as compared to 10 months in sorafenib arm (HR=1.31, 95% CI: 1.13-1.52, P=0.0019). Axitinib and regorafenib, which inhibit similar molecular targets to both sunitinib and sorafenib but exhibit a slightly different spectrum of toxicities, are now being evaluated as monotherapy after progression on sorafenib (www.clinicaltrials.gov NCT01334112, NCT01273662, NCT01210495, and NCT01774344).
Brivanib, a dual inhibitor of VEGFR and FGFR, demonstrated modest antitumor activity in both treatment-naïve and those patients who had failed prior anti-angiogenic therapy in two separate phase II studies (59,60). Based on these data, a large randomized phase III study compared brivanib to sorafenib in patients with systemic treatment-naïve, advanced HCC (61). This non-inferiority trial did not meet its primary endpoint; median overall survival with brivanib treatment was 9.5 vs. 9.9 months with sorafenib (HR=1.06, 95% CI: 0.93-1.22, P=0.3730). Albeit, antitumor activity and disease control rates were similar between each group. A randomized phase III study of brivanib after progression of disease on sorafenib versus best supportive care also failed to meet its primary endpoint of improved overall survival (62).
Linifanib, a selective inhibitor of VEGFR and PDGFR (63), also failed to improve upon the modest survival advantage of sorafenib (64). Early efficacy data were encouraging (65); however, these results did not translate into success in a large multicenter, randomized, phase III study of sorafenib versus linifanib as a first-line therapy for advanced HCC (64). Patient composition was similar to prior pivotal studies. Failing to meet the both pre-specified endpoints of superiority and non-inferiority, the median overall survival for linifanib was 9.1 vs. 9.8 months for sorafenib (HR=1.046, 95% CI: 0.896-1.221). A higher proportion of patients attained an objective response on linifanib (13% vs. 6.9%); however, serious adverse events were more common in this cohort than compared with sorafenib.
Cediranib, vandetanib, pazopanib, TSU-68, vatalanib, and lenvatinib have not reached later stages of clinical development. Cediranib, a pan-VEGFR inhibitor, has been associated with a high incidence of toxicity with minimal efficacy (66,67). Vandetanib, a small molecule inhibitor that blocks signaling through VEGFR and EGFR, is tolerable but has limited clinical activity (68). Pazopanib (69), TSU-68 (70), vatalanib (71), and lenvatinib (72) block VEGFR and other targets. Currently, these agents have an established safety profile, modest efficacy, and represent an important area of continued investigation.
Monoclonal antibodies
Over 20 separate clinical trials have assessed or are assessing bevacizumab, a monoclonal antibody directed against VEGF, in patients with advanced HCC. Evaluated regimens include monotherapy and combination therapy with chemotherapy, targeted agents, and embolization procedures. In general, completed studies have reported higher response rates than those observed with RTK inhibitors; however, adverse events such as arterial/venous thrombotic events and variceal hemorrhage (some fatal) are more common. A phase II study of bevacizumab monotherapy at two different doses in patients with advanced, liver-limited HCC demonstrated an objective response rate of 13% in 39 evaluable patients, with one patient obtaining a complete response (73). Grade 3 or 4 hypertension, hemorrhage and thrombosis occurred in 15%, 11% and 6% of the study group, respectively. One fatal esophageal hemorrhage due to varices occurred early in the course of the study. Subsequently, prophylactic variceal treatment was required prior to study enrollment. A second phase II study in advanced HCC with extrahepatic disease observed similar efficacy (ORR 14%) with bevacizumab monotherapy (74). It has not advanced to later stage development due to safety concerns regarding bleeding.
The addition of cytotoxic chemotherapy or targeted therapy to bevacizumab may augment antitumor activity. Response proportions (CR + PR) with various cytotoxic combinations range from 9-20%, with disease control rates reportedly as high as 78% (75-77). Bevacizumab and erlotinib may offer enhanced antitumor activity with a response rate of 24% and favorable patient outcomes with a median overall survival of 13.7 months (78,79). These results were not corroborated is a second study that reported minimal activity in a comparable patient population with similar disease assessment parameters and an identical dosing schedule (80). This observation serves to illustrate the heterogeneous nature of HCC and the potential for subtle differences in patient specific factors (i.e., disease burden, Child-Pugh class, etiologic factor) to either cloud interpretation of early stage trials or, as in the case of etiologic factor, potentially influence responsiveness to therapy. As seen above, it is also possible that erlotinib adds little to the effects of anti-angiogenic therapy. To clarify this issue, a multicenter, randomized phase II trial of bevacizumab combined with erlotinib (www.clinicaltrials.gov NCT00881751) versus sorafenib monotherapy is ongoing. Several other additional phase II studies are evaluating bevacizumab with sorafenib, everolimus, temsirolimus, and other treatment modalities.
Ramucirumab, a monoclonal antibody blocking VEGFR-2, was recently assessed in a phase II study comprised of 43 patients with systemic treatment-naïve advanced HCC. The majority of study participants had extrahepatic disease with excellent hepatic function. The median progression-free survival was 4.3 months with a disease control rate was 50% (7% of patients had a partial response). The agent was tolerable, but like bevacizumab, severe hypertension and hemorrhage with drug-related deaths were reported. Based on these data a randomized phase III study of ramucirumab versus best supportive care in the second line setting is ongoing (www.clinicaltrials.gov NCT01140347). Several other novel anti-angiogenic monoclonal antibodies are entering early stage development in HCC (81). Such agents may offer a more favorable safety profile, with a lower incidence of hemorrhage, which might be ideal in the HCC patient population.
Critical questions in targeting angiogenic pathways
Several important considerations remain in the treatment of this heterogeneous malignancy and for future drug development. Perhaps the most critical question is to define (if possible) the mechanistic basis for the antitumor activity of sorafenib in HCC. As discussed above, three drugs, which were perceived to be more potent and precise inhibitors of angiogenic pathways than sorafenib, failed to demonstrate greater efficacy in the clinical setting. In addition to directly interrogating patient tumor samples, there are renewed efforts to develop preclinical animal models that adequately recapitulate the features of human disease (i.e., etiologic factor, cirrhotic background, etc.). Such approaches will be important for a mechanistic understanding of angiogenesis and translating basic science breakthroughs to the clinic and vise-versa.
Establishing biomarkers of responsiveness is also a priority. Molecular sub-categorization of tumors will identify the biologic profile that might make a patient’s tumor more susceptible to a specific targeted therapy. Thus far, these attempts have been unsuccessful for sorafenib. Pretreatment serum-based response surrogates, such as VEGF, VEGFR-1, VEGFR-2, VEGFR-3, Ang-2, FGF, and several cytokines are not predictive of benefit to anti-angiogenic therapy (82). Trends toward enhanced survival from sorafenib were observed in patients with high circulating c-KIT or low hepatocyte growth factor (HGF, the ligand for c-MET) concentration at baseline. Oncogenic pathway activation as assessed by pretreatment phosphorylated-ERK, the downstream effecter of the MAPK pathway, was associated with longer time to progression on sorafenib (3). In contrast, activation of the transcriptional regulator c-Jun is associated with a poor response to sorafenib (83). These observations obviously require further validation and clarification. Other areas of intense biomarker exploration include the study of circulating tumor cells, HCC gene expression profiles, and importantly the application of next-generation sequencing technologies to define cancer genotypes that are more likely to response to targeted therapy (84,85).
Finally, defining the optimal method of radiographic assessment in HCC will be critical to assess early efficacy in phase I and II clinical trials. Thus far, anti-angiogenic therapy appears to suppress growth and disrupt the vasculature, but does not yielded dramatic tumor shrinkage. Clinical benefit occurs without tumor response. Thus, standard Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, which assesses the sum of one-dimensional measurement in multiple target lesions, may not adequately reflect the cytostatic effect of anti-angiogenic therapy on tumor viability (86). New response assessment tools have been developed to incorporate the concept of tumor viability, reflected by tissue density due to vascular enhancement. Modified RECIST incorporate decreased intra-tumoral enhancement to define a response. Limited data are available to indicate that this approach, which was never prospectively validated, is a superior surrogate to RECIST in the metastatic setting in response to anti-angiogenic therapy (87). Other proposed schemas include the ratio of tumor necrosis to tumor volume (41), volumetric measurement (86), and the application of functional MRI imaging such as dynamic-contrast enhanced (DCE), blood oxygen level dependent (BOLD), diffusion weighting, and image subtraction to assess for tumor response (41). Large prospective studies evaluating these techniques will be required before implementation of global standard.
Selected therapuetic strategies in late stage drug development
Given the multitude of drugs under evaluation in early stage clinical trials or with early safety and modest efficacy data available, an exhaustive review of each agent or each agent class will be forgone and the remaining discussion will focus on those agents that are currently under investigation on active phase III clinical trials.
Targeting the HGF/c-MET axis
Overexpression of c-MET and its ligand HGF occur in up to 80% of human HCC tumors (19). Transgenic mice that overexpress MET in hepatocytes developed HCC and inactivation of this transgene leads to tumor regression, mediated by apoptosis and growths suppression (88). Downregulation of MET in vitro using RNA interference (89), micro-RNAs (90), of transfection of NK4 (an antagonist of HGF) (91) reduces the migratory and invasive capacity of HCC cells. Finally, blocking MET with several different multi-targeted TKIs induces in vitro HCC growth suppression, cell-cycle arrest and decreased viability as well as growth suppression and survival prolongation in vivo (92). Given these data, MET has emerged as a promising target in HCC.
Tivantinib, a selective MET receptor tyrosine kinase inhibitor, was evaluated at two doses in a randomized, placebo-controlled phase II in advanced HCC patients who had progressed after first-line therapy (93). This study reported two critical findings. First, a statistically significant difference in outcomes between high-MET expressing tumors in favor of tivantinib. For patients with high MET expressing tumors, tivantinib therapy resulted in a median time to progression of 2.7 months in comparison to 1.4 months for placebo (HR=0.43, 95% CI: 0.19-0.97) and a median overall survival of 7.2 compared with 3.8 months for placebo (HR=0.38, 0.18-0.81). Importantly, no such differences between the agent and placebo were observed in low-MET expression tumor. This strongly suggests that MET expression is a predictive biomarker for MET-directed targeted therapy in HCC. Second, in those patients on the placebo arm, high tumoral MET expression was associated with an improved overall survival when compared with low tumoral MET expression (3.8 vs. 9 months, HR=2.94, 95% CI: 1.16-7.43). This observation indicates that MET expression may also be prognostic in this disease. Given these data, tivantinib is being compared with placebo in double-blind, randomized phase III study in patients with advance HCC and high-MET expressing tumors in the second-line setting (clinicaltrials.gov NCT01755767).
Cabozantinib, an inhibitor of MET and VEGFR-2, has also shown promising efficacy data in a cohort of 41 patients with advanced HCC (94). In 78% of patients, tumor regression was observed by RECIST with a 5% confirmed partial response rate. Median progression-free survival for the cohort was estimated at 4.2 months. Unfortunately baseline MET expression has not been reported. A phase III study cabozantinib is in planning. Several other agents are entering HCC-specific clinical trials, and these include oral MET inhibitors such as foretinib, golvatinib and INC280, MET blocking monoclonal antibodies, and novel combination strategies.
Targeting the mammalian target of rapamycin pathway
The mTOR pathway plays a critical role in hepatocarcinogenesis, and in xenograft mouse models, blockade of this pathway results in HCC growth suppression and lengthening of survival (20). These observations, as well as retrospective data indicating enhanced survival among patients receiving sirolimus immunosuppression following liver transplantation for HCC, piqued interest in developing these compounds in this disease. A phase I/II study of everolimus established that 10 mg daily was a safe dose (95). The phase II portion, a two-stage efficacy design, did not meet its pre-specified boundary for expansion to the second stage. Of 25 evaluable patients, 1 (4%) had a partial response and 10 (40%) had stable disease. Median time to progression was 3.9 months and median overall survival was 8.4 months. Presently, everolimus is being investigated in the second line setting after sorafenib failure in the phase III, randomized, placebo-controlled EVOLVE-1 study (www.clinicaltirals.gov NCT01035229). Temsirolimus, AZD8055, as well as multiple combination strategies are ongoing.
Targeting metabolic pathways
The biosynthesis of the nonessential amino acid arginine occurs as part of the urea cycle and is dependent upon the enzymes argininosuccinate synthetase and argininosuccinate lyase. Messenger RNA encoding argininosuccinate synthetase is not present in subsets of hepatocellular carcinomas, therefore arginine must be extracted from the circulation (96). Pegylated arginine deiminase (ADI-PEG 20) is an arginine degrading enzyme isolated from Mycoplasma that is formulated with polyethylene glycol (molecular weight 20 kilodalton). In preclinical models, ADI-PEG 20 decreases HCC cell viability at low nanomolar concentrations, reduces serum arginine levels to undetectable levels, and prolongs survival in HCC xenograft mouse models. A phase I/II study demonstrated an excellent safety profile in a patient population comprised with a high burden of disease and impaired hepatic function (~49% study population Child Pugh B or C) (97). The most common events were injection site reactions and isolated lab abnormalities such as elevated fibrinogen. Of 19 patients evaluable, 2 (10.5%) had complete response, 7 (36.8%) had a partial response and 7 (36.8%) had stable disease. The duration of response ranged from 37 to >680 days. Two subsequent randomized phase II studies that compared escalating doses demonstrated less marked antitumor efficacy (98,99). Glazer and colleagues reported a disease control rate of 63.1% and 2.6% objective response rate and a median overall survival of 11.4 months (98). This exclusively European patient population was composed predominately of HCV-associated (79%) HCC confined to the liver (84%) with otherwise excellent hepatic function (81%). In contrast, Yang and colleagues tested the agent in a heavily pretreated Asian population with HBV-associated (69%) extrahepatic (58%) hepatocellular carcinoma. In this study, no objective responses were noted and the median overall survival was 7.3 months. Currently, a double blind placebo controlled study of ADI-PEG 20 after prior systemic therapy is ongoing (www.clinicaltrials.gov NCT01287585).
Conclusions and future directions
Despite the availability of sorafenib as a standard of care for HCC, there is a substantial need to enhance the armamentarium of therapies in the metastatic setting. Presently, the global standard of care for a patient presenting with metastatic hepatocellular carcinoma is either clinical trial enrollment or sorafenib monotherapy. Although several, high-profile, phase III clinical trials have failed to improve on the current standard, the pipeline for drug development is robust, preliminary phase II data are promising for several agents, and the international research community is committed to continued collaboration to understand this complex disease. In the laboratory, interrogation of HCC genome may isolate novel targets. It is also likely that more trials will attempt to select molecular profiles that are predicted to respond to specific targeted therapy, as in the case of MET inhibition. Looking forward, there will certainly be a greater attention to immune based therapy. Tremelimumab, a CTLA-4 blocking antibody, demonstrated durable disease control in a recent phase II study in addition to exhibiting antiviral activity (100). Several trials evaluating other immune checkpoint modulators (i.e., anti-PD-1 and anti-PDL1) are ongoing or are being planned. Engineered viral stains, termed oncolytic immunotherapeutics, are capable of selectively targeting tumors by inducing both viral replication-dependent tumor death and tumor-specific immunity (101). This approach has shown promising activity as well. Finally, efforts will continue to target the WNT pathway, which is heavily disrupted in HCC. Hopefully, the international field will continue to witness meaningful progress for the treatment of patients with metastatic hepatocellular carcinoma.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Abou-Alfa GK. Hepatocellular carcinoma: molecular biology and therapy. Semin Oncol 2006;33:S79-83. [PubMed]
- Zhu AX, Duda DG, Sahani DV, et al. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol 2011;8:292-301. [PubMed]
- Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:4293-300. [PubMed]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [PubMed]
- Ang C, O’Reilly EM, Abou-Alfa GK. Targeted agents and systemic therapy in hepatocellular carcinoma. Recent Results Cancer Res 2013;190:225-46. [PubMed]
- Hsu HC, Jeng YM, Mao TL, et al. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol 2000;157:763-70. [PubMed]
- Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer 2001;92:136-45. [PubMed]
- Kim YD, Park CH, Kim HS, et al. Genetic alterations of Wnt signaling pathway-associated genes in hepatocellular carcinoma. J Gastroenterol Hepatol 2008;23:110-8. [PubMed]
- Guichard C, Amaddeo G, Imbeaud S, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet 2012;44:694-8. [PubMed]
- Fujimoto A, Totoki Y, Abe T, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet 2012;44:760-4. [PubMed]
- Huang J, Deng Q, Wang Q, et al. Exome sequencing of hepatitis B virus-associated hepatocellular carcinoma. Nat Genet 2012;44:1117-21. [PubMed]
- Moinzadeh P, Breuhahn K, Stützer H, et al. Chromosome alterations in human hepatocellular carcinomas correlate with aetiology and histological grade--results of an explorative CGH meta-analysis. Br J Cancer 2005;92:935-41. [PubMed]
- Merle P, de la Monte S, Kim M, et al. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology 2004;127:1110-22. [PubMed]
- Boyault S, Rickman DS, de Reyniès A, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007;45:42-52. [PubMed]
- Lachenmayer A, Alsinet C, Savic R, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res 2012;18:4997-5007. [PubMed]
- Wang Y, Han C, Lu L, et al. Hedgehog signaling pathway regulates autophagy in human hepatocellular carcinoma cells. Hepatology 2013;58:995-1010. [PubMed]
- Villanueva A, Alsinet C, Yanger K, et al. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology 2012;143:1660-9.e7.
- Awuah PK, Nejak-Bowen KN, Monga SP. Role and regulation of PDGFRα signaling in liver development and regeneration. Am J Pathol 2013;182:1648-58. [PubMed]
- Chu JS, Ge FJ, Zhang B, et al. Expression and prognostic value of VEGFR-2, PDGFR-β, and c-Met in advanced hepatocellular carcinoma. J Exp Clin Cancer Res 2013;32:16. [PubMed]
- Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 2008;135:1972-83,1983.e1-11.
- Huynh H, Nguyen TT, Chow KH, et al. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol 2003;3:19. [PubMed]
- Wiesenauer CA, Yip-Schneider MT, Wang Y, et al. Multiple anticancer effects of blocking MEK-ERK signaling in hepatocellular carcinoma. J Am Coll Surg 2004;198:410-21. [PubMed]
- Lee HC, Tian B, Sedivy JM, et al. Loss of Raf kinase inhibitor protein promotes cell proliferation and migration of human hepatoma cells. Gastroenterology 2006;131:1208-17. [PubMed]
- Wang C, Cigliano A, Delogu S, et al. Functional crosstalk between AKT/mTOR and Ras/MAPK pathways in hepatocarcinogenesis: Implications for the treatment of human liver cancer. Cell Cycle 2013;12:1999-2010. [PubMed]
- Huang J, Zhang X, Tang Q, et al. Prognostic significance and potential therapeutic target of VEGFR2 in hepatocellular carcinoma. J Clin Pathol 2011;64:343-8. [PubMed]
- Li T, Zhu Y, Qin CY, et al. Expression and prognostic significance of vascular endothelial growth factor receptor 1 in hepatocellular carcinoma. J Clin Pathol 2012;65:808-14. [PubMed]
- von Marschall Z, Cramer T, Höcker M, et al. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut 2001;48:87-96. [PubMed]
- Desbois-Mouthon C, Baron A, Blivet-Van Eggelpoël MJ, et al. Insulin-like growth factor-1 receptor inhibition induces a resistance mechanism via the epidermal growth factor receptor/HER3/AKT signaling pathway: rational basis for cotargeting insulin-like growth factor-1 receptor and epidermal growth factor receptor in hepatocellular carcinoma. Clin Cancer Res 2009;15:5445-56. [PubMed]
- Shao RX, Otsuka M, Kato N, et al. Acyclic retinoid inhibits human hepatoma cell growth by suppressing fibroblast growth factor-mediated signaling pathways. Gastroenterology 2005;128:86-95. [PubMed]
- Hu TH, Huang CC, Lin PR, et al. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer 2003;97:1929-40. [PubMed]
- Majumdar A, Curley SA, Wu X, et al. Hepatic stem cells and transforming growth factor β in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2012;9:530-8. [PubMed]
- Huang S, Zhang F, Miao L, et al. Lentiviral-mediated Smad4 RNAi induced anti-proliferation by p16 up-regulation and apoptosis by caspase 3 down-regulation in hepatoma SMMC-7721 cells. Oncol Rep 2008;20:1053-9. [PubMed]
- Azechi H, Nishida N, Fukuda Y, et al. Disruption of the p16/cyclin D1/retinoblastoma protein pathway in the majority of human hepatocellular carcinomas. Oncology 2001;60:346-54. [PubMed]
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science 2013;339:957-9. [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [PubMed]
- Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 2005;23:965-72. [PubMed]
- Furuse J, Ishii H, Nakachi K, et al. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci 2008;99:159-65. [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [PubMed]
- Abou-Alfa GK. Selection of patients with hepatocellular carcinoma for sorafenib. J Natl Compr Canc Netw 2009;7:397-403. [PubMed]
- Cheng A, Kang Y, Lin D, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2011;29:abstr 4000.
- Giambartolomei S, Covone F, Levrero M, et al. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the Hepatitis C Virus (HCV) core protein. Oncogene 2001;20:2606-10. [PubMed]
- Himmelsbach K, Sauter D, Baumert TF, et al. New aspects of an anti-tumour drug: sorafenib efficiently inhibits HCV replication. Gut 2009;58:1644-53. [PubMed]
- Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA 2010;304:2154-60. [PubMed]
- Alavi AS, Acevedo L, Min W, et al. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on Raf-1-mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res 2007;67:2766-72. [PubMed]
- McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 2006;46:249-79. [PubMed]
- Hoffmann K, Franz C, Xiao Z, et al. Sorafenib modulates the gene expression of multi-drug resistance mediating ATP-binding cassette proteins in experimental hepatocellular carcinoma. Anticancer Res 2010;30:4503-8. [PubMed]
- Zaanan A, Williet N, Hebbar M, et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol 2013;58:81-8. [PubMed]
- Assenat E, Boige V, Thézenas S, et al. Sorafenib (S) alone versus S combined with gemcitabine and oxaliplatin (GEMOX) in first-line treatment of advanced hepatocellular carcinoma (HCC): final analysis of the randomized phase II GONEXT trial (UNICANCER/FFCD PRODIGE 10 trial). J Clin Oncol 2013,31: abstr 4028.
- Sieghart W, Pinter M, Dauser B, et al. Erlotinib and sorafenib in an orthotopic rat model of hepatocellular carcinoma. J Hepatol 2012;57:592-9. [PubMed]
- Duran I, Hotté SJ, Hirte H, et al. Phase I targeted combination trial of sorafenib and erlotinib in patients with advanced solid tumors. Clin Cancer Res 2007;13:4849-57. [PubMed]
- Quintela-Fandino M, Le Tourneau C, Duran I, et al. Phase I combination of sorafenib and erlotinib therapy in solid tumors: safety, pharmacokinetic, and pharmacodynamic evaluation from an expansion cohort. Mol Cancer Ther 2010;9:751-60. [PubMed]
- Zhu AX, Rosmorduc O, Evans J, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with hepatocellular carcinoma (HCC), ESMO 2012, Vienna, 2012.
- Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003;9:327-37. [PubMed]
- Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol 2009;27:3027-35. [PubMed]
- Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol 2009;10:794-800. [PubMed]
- Koeberle D, Montemurro M, Samaras P, et al. Continuous Sunitinib treatment in patients with advanced hepatocellular carcinoma: a Swiss group for clinical cancer research (SAKK) and Swiss association for the study of the liver (SASL) multicenter phase II trial (SAKK 77/06). Oncologist 2010;15:285-92. [PubMed]
- Finn RS, Kang YK, Mulcahy M, et al. Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2012;18:2090-8. [PubMed]
- Park JW, Finn RS, Kim JS, et al. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2011;17:1973-83. [PubMed]
- Johnson P, Qin S, Park JW. Brivanib versus sorafenib as first-line therapy in patients with unresectable advanced hepatocellular carcinoma: results from the phase 3 BRISK-FL study. 3rd annual meeting of the American association for the study of liver disease, Boston, 2012.
- Llovet JM, Decaens T, Raoul JL, et al. Brivanib versus placebo in patients with advanced hepatocelluar carcionam who fialed or were intolerant to sorafenib: results from the phase 3 BRISK-PS study. J Hepatol 2012;56:S549.
- Albert DH, Tapang P, Magoc TJ, et al. Preclinical activity of ABT-869, a multitargeted receptor tyrosine kinase inhibitor. Mol Cancer Ther 2006;5:995-1006. [PubMed]
- Cainap C, Qin S, Huang WT, et al. Phase III trial of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2012;30:abstr 249.
- Toh HC, Chen PJ, Carr BI, et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer 2013;119:380-7. [PubMed]
- Alberts SR, Fitch TR, Kim GP, et al. Cediranib (AZD2171) in patients with advanced hepatocellular carcinoma: a phase II North Central Cancer Treatment Group Clinical Trial. Am J Clin Oncol 2012;35:329-33. [PubMed]
- Zhu AX, Ancukiewicz M, Supko JG, et al. Efficacy, safety, pharmacokinetics, and biomarkers of cediranib monotherapy in advanced hepatocellular carcinoma: a phase II study. Clin Cancer Res 2013;19:1557-66. [PubMed]
- Hsu C, Yang TS, Huo TI, et al. Vandetanib in patients with inoperable hepatocellular carcinoma: a phase II, randomized, double-blind, placebo-controlled study. J Hepatol 2012;56:1097-103. [PubMed]
- Yau T, Chen PJ, Chan P, et al. Phase I dose-finding study of pazopanib in hepatocellular carcinoma: evaluation of early efficacy, pharmacokinetics, and pharmacodynamics. Clin Cancer Res 2011;17:6914-23. [PubMed]
- Kanai F, Yoshida H, Tateishi R, et al. A phase I/II trial of the oral antiangiogenic agent TSU-68 in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol 2011;67:315-24. [PubMed]
- Koch I, Baron A, Roberts S, et al. Influence of hepatic dysfunction on safety, tolerability, and pharmacokinetics (PK) of PTK787/ZK 222584 in patients (Pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol 2005;23:abstr 4134.
- Mitsunaga S, Ikeda M, Ueno H, et al. Phase I/II study of lenvatinib (E7080), a multitargeted tyrosine kinase inhibitor, in patients (pts) with advanced hepatocellular carcinoma (HCC): phase I results. J Clin Oncol 2012;30:abstr 231.
- Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 2008;26:2992-8. [PubMed]
- Boige V, Malka D, Bourredjem A, et al. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist 2012;17:1063-72. [PubMed]
- Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:1898-903. [PubMed]
- Hsu CH, Yang TS, Hsu C, et al. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer 2010;102:981-6. [PubMed]
- Sun W, Sohal D, Haller DG, et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011;117:3187-92. [PubMed]
- Thomas MB, Morris JS, Chadha R, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol 2009;27:843-50. [PubMed]
- Kaseb AO, Garrett-Mayer E, Morris JS, et al. Efficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: final results of a phase II trial. Oncology 2012;82:67-74. [PubMed]
- Philip PA, Mahoney MR, Holen KD, et al. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer 2012;118:2424-30. [PubMed]
- Rosen LS, Hurwitz HI, Wong MK, et al. A phase I first-in-human study of TRC105 (Anti-Endoglin Antibody) in patients with advanced cancer. Clin Cancer Res 2012;18:4820-9. [PubMed]
- Llovet JM, Peña CE, Lathia CD, et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2012;18:2290-300. [PubMed]
- Hagiwara S, Kudo M, Nagai T, et al. Activation of JNK and high expression level of CD133 predict a poor response to sorafenib in hepatocellular carcinoma. Br J Cancer 2012;106:1997-2003. [PubMed]
- Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008;359:1995-2004. [PubMed]
- Garraway LA, Verweij J, Ballman KV. Precision oncology: an overview. J Clin Oncol 2013;31:1803-5. [PubMed]
- Sharma MR, Maitland ML, Ratain MJ. RECIST: no longer the sharpest tool in the oncology clinical trials toolbox---point. Cancer Res 2012;72:5145-9; discussion 5150. [PubMed]
- Kawaoka T, Aikata H, Murakami E, et al. Evaluation of the mRECIST and α-fetoprotein ratio for stratification of the prognosis of advanced-hepatocellular-carcinoma patients treated with sorafenib. Oncology 2012;83:192-200. [PubMed]
- Wang R, Ferrell LD, Faouzi S, et al. Activation of the met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol 2001;153:1023-34. [PubMed]
- Salvi A, Arici B, Portolani N, et al. In vitro c-met inhibition by antisense RNA and plasmid-based RNAi down-modulates migration and invasion of hepatocellular carcinoma cells. Int J Oncol 2007;31:451-60. [PubMed]
- Salvi A, Sabelli C, Moncini S, et al. MicroRNA-23b mediates urokinase and c-met downmodulation and a decreased migration of human hepatocellular carcinoma cells. FEBS J 2009;276:2966-82. [PubMed]
- Heideman DA, Overmeer RM, van Beusechem VW, et al. Inhibition of angiogenesis and HGF-cMET-elicited malignant processes in human hepatocellular carcinoma cells using adenoviral vector-mediated NK4 gene therapy. Cancer Gene Ther 2005;12:954-62. [PubMed]
- Huynh H, Ong R, Soo KC. Foretinib demonstrates anti-tumor activity and improves overall survival in preclinical models of hepatocellular carcinoma. Angiogenesis 2012;15:59-70. [PubMed]
- Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol 2013;14:55-63. [PubMed]
- Verslype C, Cohn AL, Kelley RK, et al. Activity of cabozantinib (XL184) in hepatocellular carcinoma: results from a phase II randomized discontinuation trial (RDT). J Clin Oncol 2012;30:abstr 4007.
- Zhu AX, Abrams TA, Miksad R, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer 2011;117:5094-102. [PubMed]
- Ensor CM, Holtsberg FW, Bomalaski JS, et al. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res 2002;62:5443-50. [PubMed]
- Izzo F, Marra P, Beneduce G, et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol 2004;22:1815-22. [PubMed]
- Glazer ES, Piccirillo M, Albino V, et al. Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. J Clin Oncol 2010;28:2220-6. [PubMed]
- Yang TS, Lu SN, Chao Y, et al. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer 2010;103:954-60. [PubMed]
- Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81-8. [PubMed]
- Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 2013;19:329-36. [PubMed]


