
Challenges and insights of early phase oncology drug development in the Asia-Pacific region
Introduction
The advent of globalization has led to significant changes in the biomedical field over the past two decades. Traditionally, phase 1 clinical trials or First-in-Human (FIH) trials are the first step in clinical drug development and are primarily designed to evaluate the safety of new agents and the recommended dose for further definitive testing in a small group of participants. This may have been an undirected approach involving patients of various types of cancer. The commonality between subjects participating through such an approach is that they commonly had a reasonable performance status, adequate haematological and biochemical reserves, and have exhausted otherwise approved treatments for their condition. Whilst taking such a broad-based approach in terms of patients’ recruitment may potentially increase the rapidity of subjects’ recruitment as it casts a wider net for eligible patients and determination of a recommended phase 2 dose for further testing, it does not reliably provide any efficacy information. This limitation has become more apparent in the era of advanced genomics and targeted therapies, whereby many a times improved efficacy of a therapy is not necessarily guaranteed by a higher or near toxic dose, but rather by interfering with known molecular pathways responsible for carcinogenesis in these specific diseases. Additional pre-requisites on the patients’ disease phenotype or genotype, some of which may not be evenly distributed globally, as well as the need for a larger pool of potentially suitable subjects, coupled with increasing healthcare demands in the region, have all contributed to a surge of interest in conducting phase 1 oncology clinical trials in the Asia-Pacific.
Reliable efficacy data can now be read out from phase 1 clinical trials. It is essential to conduct clinical research in populations proportional to the potential uses of the products after approval, even from the earliest drug development process. Other justifications for doing early clinical trials in Asia is a result of our increasing recognition of ethnic and genetic factors that may play pivotal parts in the variability of subjects’ responses to a medication and adverse drug reactions. There is thus a need to involve Asian patients from the early phases of drug development.
In this review, we shall discuss some of the challenges and opportunities in conducting early phase oncology trials in Asia, as well as introduce some of the concerted efforts that several leading institutions from the region have organized to form the essential infrastructure required to further advance this field in the region.
Challenges of early phase oncology drug development in Asia
Geographic and ethnic diversity
The Asia-Pacific region encompasses a large part of the world in or near the Western Pacific Ocean. Depending on which context, this region typically includes much of East Asia, South Asia, Southeast Asia and Oceania. Whilst this region is often described as a ‘singularity’ in a geographical context, the ethnic, cultural and economic differences both within and between countries in the region cannot be more different. Populations of individual nations within this area range from just over three-hundred thousand in the Maldives to over 1.3 billion in China. Asia is also a region rich in ethnic and cultural diversity (1). As an example, over 2,000 ethnic groups, each with their distinct cultural heritage make up the population of India, and over 50 ethnicities make up the population of China.
Healthcare disparities
The difference between lowest and highest gross domestic product (GDP) per capita in the region is over 100-fold (in USD: Nepal 730 vs. Macau 73,187) (2). Total health expenditure (THE) per capita also shows significant variation across the countries, with the highest being Australia versus the lowest in the Lao People’s Democratic Republic (USD 4,357 vs. 35.5) (2). Moreover, THE as a share of GDP is highest in high-income countries such as Australia (10.0%), New Zealand (9.4%) and Republic of Korea (7.4%) (2). However, interestingly, there are exceptions to this with Singapore and Brunei Darussalam spending less than the average 9% of GDP on health amongst all countries (4.3% and 1.3% respectively) (2). The share of government in total health spending varies from as high as 93.8% in Brunei Darussalam to as low as 18.9% in China (2). ‘Out-of-pocket spending’ accounts for a much greater share of health expenditures in lower-middle income than high-income countries. Some countries have social health insurance schemes which constitute a significant portion of THE. Overall, provision and accessibility to healthcare and its infrastructure is vastly different between different communities in the region.
Drug approval timelines in Asia
Delay in approval and access of new anticancer therapies in Asia compared has led to increased interest in simultaneous global clinical development to be inclusive of Asia. Previously, this delay often arises in part from specific regulatory requirements for local patient data across Asia (e.g., Japan, China and Taiwan). As an example, to receive approval in Japan from the Pharmaceuticals and Medical Devices Agency (PMDA), clinical safety and efficacy data from Japanese patients must be part of the submission (3), although steps have been taken by these individual healthcare regulatory authorities to streamline the process for drug approval. To speed up the approval process by taking advantage of clinical data obtained from outside Japan, the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) issued a set of recommendations in 1998 (4). This guidance, known as ICH E5, discusses the potential sources of differential pharmacokinetics (PK), pharmacodynamics (PD), and clinical responses in different ethnic populations, and describes the circumstances under which data from clinical trials from outside jurisdictions can be used for approval within a health system. The development strategy that emerged after publication of ICH E5 is known as a “bridging strategy,” and has been conceptually applied throughout Asia (5).
Despite the ICH E5 guidance, delays in drug development still exist in Asia. As an example, an evaluation of factors contributing to approval lag in Japan has been the subject of many recently published analyses (6-8). In a recent review of oncology drugs approved between 2001 and 2014 in Japan and also approved in the United States (US), a steady decline in approval lag over this time period has been reported, with the median approval lag for drugs approved in 2014 being 9.4 months, which is still meaningful but substantially lower than that for the overall set of drugs approved during 2001–2014 (29.2 months) (8).
However, a global approach to drug development also faces several key challenges. Regional differences in drug exposures, safety and efficacy are complex and multifactorial. In recent reviews of drugs that have been approved in Japan from 2001–2009, the doses approved for use in the West were higher in 32% of drugs across all therapeutic classes (9,10). Based on a review of 40 oncology drugs approved in Japan between 2001 and 2013, the approved doses in Japan and the US or European Union (EU) was different for 5 (12.5%) of these 40 drugs (11). These differences can be accounted by the narrow therapeutic window of anticancer drugs and reconfirms the need and importance of characterization of PKs and PDs within Asia. A concerted effort is required to reduce the delays in drug development and approval. It is advisable to adopt a more global perspective in early phase clinical drug development and to reduce the number of redundant trials, improve overall trial designs, and obtain clinical data that can be used to support approval in Asian countries. Past experiences have confirmed that approval delays are shortened in compounds of which the development strategies incorporated a global or multiregional approach.
Early phase trial infrastructure
There are a variety of push-and-pull factors which contribute to the success of early phase clinical trial programmes. This applies to both oncology and non-oncology related clinical trials, including (I) presence of clinical and translational research expertise; (II) good accessibility both in terms of allowing subjects/patients to attend clinical trial centres as well as for investigators to have ‘access’ to potential subjects/patients; and (III) solid standard-of-care healthcare provisions to ensure that adequate coverage can be provided for subjects/patients who may run into issues with toxicities, especially in FIH clinical trials. Aside from technical capabilities and population catchments at the local site level, the regulatory environment for conducting clinical trials as well as for drug registration and enlistment are significant ‘pull-factors’ which cannot be ignored. Out of the over 40 economies in the region, over 20 healthcare systems have been relatively more active in terms of promulgating and implementing regulations that impact the biopharmaceutical industry and advance drug development.
Logistics for initiating clinical trials in various jurisdictions are highly variable. Regulatory requirements, speed of subjects/patients’ enrollment, and difference in standard-of-care clinical practice all have implications on the practicality of conducting specific clinical trials in a region will inevitably also have significant implications on the timeline for clinical trial completion. Given the increasing globalized approach of conducting multi-national early phase clinical trials, the discrepancies within the Asia-Pacific region may not only delay regional, but overall global registration timelines.
Opportunities for early phase oncology drug development in Asia
Phase I expertise in Asia
Despite some of the aforementioned challenges, several institutions in Asia have invested in developing phase I capabilities. While phase II and III trials in Asian-prevalent cancers such as hepatocellular carcinoma, nasopharyngeal cancer and gastric cancer have successfully capitalized on the sizable patient population and unique demographics in Asia, the advent of biomarker driven clinical trials necessitated additional infrastructure to conduct such studies. EGFR mutant non-small cell lung cancer (NSCLC) represents one of the examples of a molecular selected population, where novel therapeutic approaches remain actively examined in early phase trials across Asian sites. With a critical mass of phase I investigators in the region, engagement in pivotal early phase trials remains crucial given how we have seen approvals granted based on small, molecularly selected patient cohorts.
Leveraging on Asia: obstacles and opportunities
Despite the large patient population, there is substantial disparity in the region for delivery of optimal health care. Within each country, outside of the major cities, there is typically minimal access to molecular screening and specialist opinions. Between countries, one of the major hurdles has been the coordination of studies across international borders—especially for investigator-initiated trials. To address this, disease specific groups have now been set up, e.g., Asian Thoracic Oncology Research Group (ATORG), whose mission is to coordinate both clinical trials and translational research in lung cancer across Asia. To date some of the studies initiated include a molecular profiling study (ATORG001), utilizing the Oncomine Focus Assay that detects 52 genes including mutation hotspots, copy number, common fusions in NSCLC. This study commenced in January 2019 and intends to capture the genomic profile of 500 NSCLC patients. Multi-centre investigator-initiated trials will also be operationalized in July 2019, using a lead sponsor and delegated sponsor model that will be coordinated out of the ATORG office. However, one of the major challenges in drug development efforts, has been the lack of “one stop” regulatory agency for the region.
Regulatory approvals and access
Although involvement in early phase studies have facilitated submissions to local health authorities in ethnically relevant patient populations, there are several inherent inefficiencies with drug approvals across Asia. As each country within Asia has its own regulatory agency and reimbursement policies, access to new therapies can vary widely across individual countries, notwithstanding the lag time of typically 12 to 18 months from initial FDA/EMEA approvals. Coupled with escalating costs of cancer medicines, there remain opportunities for trials in Asia that would be challenging to conduct in US, for example immune checkpoint inhibitor naïve NSCLC. Thus, if drug development and approvals in Asia remains status quo, one inevitable consequence would be increasing divergent standards of care with EU and US.
Whilst there is an increasing number of these healthcare agencies participating in the International Council on Harmonization (ICH) with growing interest in identifying and incorporating best practices from competent regulatory agencies, further work still needs to be done for possible harmonization and mutual recognition of clinical trial results between different health agencies to further improve the efficiency of the drug development process in the region. Unified or, at the very least, mutually recognized requirements and processes for new drug approval within the region needs to be discussed between various stakeholders. This may include, but not limited to (I) harmonization of specific standard operating procedures (SOPs) between different clinical trial sites or regulatory agencies; (II) requirements for registration and filing of investigational new drugs (INDs); (III) free-passage of clinical samples between participating clinical trial sites in different countries to further enhancement the feasibility of international translational research and (IV) harmonization of the language used in study documents, preferably in English, for ease of regional and international communication. Ultimately, the clear advantages of the Asia-Pacific region’s diversity, dynamism and large population should be harnessed to aid global drug development.
Practical strategies for enhancing Asia’s contribution to drug development
Asian Oncology Early Phase 1 Consortium (AsiaOne)
Among global markets, Asia is well positioned to be the preferred destination for oncology drug development and clinical trials, because of its speed, cost-efficiency, and the similarity in standard treatment with US/Europe. In terms of key aspect of early phase drug development focusing on East Asia, emergence of importance of efficient and strategic early drug development system in Asian region has been arisen during recent decade. Despite most of pharmaceutical sponsors are interested in the academic platform which could effectively utilize their early phase drug development program in Asia, there were no pragmatic official transverse consortium which dedicatedly deals with oncology early phase 1 clinical trials in Asia.
Asian Early Phase 1 Oncology Drug Development Consortium (AsiaOne) formed under the conclusion of the agreement on September 14, 2017 across early phase new drug development institutions (key oncology phase 1 centers) in China (Hong Kong), Japan, Republic of Korea, Singapore and Taiwan, to form the Asian Oncology Early Phase 1 Consortium, to drive the momentum towards international collaborative phase 1 clinical trials across Asia, and to realize efficient clinical development of early phase oncology drugs serving the region (Figure 1).
By promoting multinational collaborative clinical trials across Asia initiated by pharmaceutical companies with this platform, multinational investigator-initiated clinical trials coordinated by Asian Oncology Early Phase 1 investigators, collaboration with early phase drug development divisions of pharmaceutical companies, and personnel exchange between member institutions, AsiaOne aims to advance early phase drug development in Asia, in particular genomic medicine and treatments for rare cancers. Asia needs to take the initiative in developing drugs to treat cancers prevalent in the region, namely those of Asian specific cancers like as the stomach, liver, and biliary tract. Drug discovery efforts initiated in Asia, rather than traditional developers of North America and Europe, are needed to develop treatments suitable for Asian populations and international collaborative clinical trials by a premier group of leading institutions of Asian countries will play a significant role.
The outlines for collaborations are as follows:
- Collaborative framework of leading early phase new drug development institutions in Asia;
- Opportunities for international collaborative clinical trials (both pharmaceuticals-sponsored and investigator-initiated clinical trials) shared among member institutions, in early phase clinical studies (phase 1), and in collaborative research in Asia;
- Collective, coordinated effort inviting pharmaceutical companies to utilize the Consortium.
Looking ahead, there are significant potentials for the AsiaOne platform. Specifically, we believe that the establishment of AsiaOne will encourage (I) advancement of international collaborative clinical trials of early phase new drug development in Asia initiated by Western and Asian pharmaceutical companies; and (II) create an environment for collaborative investigator-initiated clinical trials and research initiated by Asian Oncology Early Phase 1 Consortium investigators. This will also help (III) strengthen the partnership between early phase new drug development divisions of pharmaceutical companies and major early phase new drug development institutions in Asia. AsiaOne will also form a bridge early and late phase oncology drug development in Asia. Looking ahead, there is a strong need for interaction promoted between young researchers and investigators, support staff of premier early phase new drug development institutions in Asia. Ultimately, the aim is to improve Asia-Pacific citizens’ health and welfare through mutual collaborations.
Achievements and limitations
Since official conclusion of the memorandum agreement on September 2017, total number of oncology phase 1 trials which involve AsiaOne sites are currently increasing and more than half of FIH phase 1 trials are global FIH trials where several AsiaOne sites participate in phase 1 trials from dose escalation part simultaneously with both US and Europe phase 1 sites (Table 1). In contrary to these current achievement, potential limitations considered to be arisen in terms of various aspects including limitation of “total one-stop services” for pharmaceutical sponsors and stakeholders including lack of central institutional review board system, one-stop study contract service across multi-regional clinical trials sites on each East Asian country.
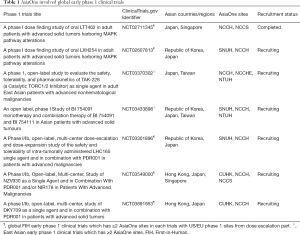
Full table
The aim of the Consortium is to promote collaborative drug development with a specific Asian focus, which includes Asian specific cancers and therapeutics suitable for Asian populations. By conducting multi-center phase 1 clinical trials between the initial five institutions, we hope to increase the awareness and interests of developmental therapeutics in oncology in the Asia-Pacific region. AsiaOne provides a platform with state-of-the-art infrastructure and a pool of research experts for both industry and academia to conduct oncology early phase clinical research.
LC-SCRUM-Asia
With recent advances in genomic analysis technology such as next generation sequencing (NGS), progress is being made in establishing cancer genomic medicine, whereby potentially effective therapies are selected based on the results of genomic analysis of each individual cancer patient. In addition to molecular targeted drugs targeting the EGFR gene mutation and the ALK fusion gene, new molecular targeted therapies have been approved in Japan: a drug targeting the ROS1 fusion gene in 2017 and a drug targeting the BRAF gene mutation in 2018. Progress is also being made in the development of a variety of treatments targeting changes in genes such as MET, RET, HER2 and NTRK, and work on establishing genomic medicine for lung cancer is expected to accelerate further in the future. However, each of these genomic alterations is rare, with a frequency of 1% to 2% of all NSCLCs, and rapid development of therapies based on clinical studies requires cooperation not only within Japan but also from other Asian countries, in order to establish a large-scale international genomic screening infrastructure in Asia.
LC-SCRUM is a nationwide genetic screening project conducted by the National Cancer Center of Japan (“NCC Japan”) in cooperation with over 200 medical institutions and pharmaceutical manufacturers across Japan. Since 2013, LC-SCRUM has performed screening of treatment target genes in over 7,000 lung cancer patients and contributed to the development of multiple novel therapeutics in Japan. Academia and pharmaceutical industries work together as a single organization to further development of new drugs and diagnostics, with the aim of establishing personalized genome medicine for advanced lung cancer. The NCC Japan announced the signing of a collaboration between its Cancer Genomic Screening Project for Individualized Medicine (“LC-SCRUM”) and Cancer Hospital in Taiwan—marking the first Asian international expansion of this groundbreaking genomic project since March 2019. Under terms of the collaboration, Taiwan has joined the SCRUM network of screening hospitals to create a regional cancer genomic screening network (“LC-SCRUM-Asia”) for lung cancer patients. LC-SCRUM-Asia strives to provide genomic screening on a regional scale and accelerate the development of innovative cancer therapies based on increasing understanding of the genetic alterations.
Conclusions
With over 60 percent of the world’s population residing in Asia and the rapidly improving economies in the region, there is inevitably increasing interests for early phase oncology drug development to take place in Asia. Whilst there may be existing limitations and controversies of provision of healthcare in some Asian countries, overall there is a trend towards rapid development and tremendous opportunities for further growth. The distinct differences in disease patterns, as well as implications of pharmacogenomics in different ethnicities all contribute to the need of Asian focused drug development platforms to contribute to global drug development efforts. As outlined above, multinational efforts are already taking place in this regard, and we look forward to further significant contributions to the global drug development arena by our Asian colleagues.
Acknowledgments
We greatly thank Dr. Koichi Goto (Chief, Department of Thoracic Oncology, National Cancer Center Hospital East) who is a representative of LC-SCRUM for information sharing of LC-SCRUM-Asia. We also thank Dr. Chia-Chi (Josh) Lin (Director of Phase 1 Center, Department of Oncology, National Taiwan University Hospital, Taipei, Taiwan) and Dr. Dong-Wan Kim (Professor, Department of Internal Medicine, Seoul National University Hospital, Seoul, Republic of Korea) for robust collaboration under Asian Oncology Early Phase 1 Consortium (AsiaOne).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- The World at Six Billion. Department of Economic and Social Affairs, United Nations. Available online: . Accessed 11 June 2019 2019.https://web.archive.org/web/20160305042434/http://www.un.org/esa/population/publications/sixbillion/sixbillion.htm
- How pharmaceutical systems are organized in Asia and the Pacific. Manila: World Health Organization Regional Office for the Western Pacific, 2018.
- Han FV, Weiss K. Regulatory Trends in Drug Development in Asia Pacific. Ther Innov Regul Sci 2018. [Crossref] [PubMed]
- Uyama Y, Shibata T, Nagai N, et al. Successful bridging strategy based on ICH E5 guideline for drugs approved in Japan. Clin Pharmacol Ther 2005;78:102-13. [Crossref] [PubMed]
- Venkatakrishnan K, Burgess C, Gupta N, et al. Toward Optimum Benefit-Risk and Reduced Access Lag For Cancer Drugs in Asia: A Global Development Framework Guided by Clinical Pharmacology Principles. Clin Transl Sci 2016;9:9-22. [Crossref] [PubMed]
- Yonemori K, Hirakawa A, Ando M, et al. The notorious "drug lag" for oncology drugs in Japan. Invest New Drugs 2011;29:706-12. [Crossref] [PubMed]
- Maeda H, Kurokawa T. Regulatory review time for approval of oncology drugs in Japan between 2001 and 2014. Considerations of changes, factors that affect review time, and difference with the United States. J Clin Pharmacol 2015;55:481-9. [Crossref] [PubMed]
- Maeda H, Kurokawa T. Recent trends for drug lag in clinical development of oncology drugs in Japan: does the oncology drug lag still exist in Japan? Int J Clin Oncol 2015;20:1072-80. [Crossref] [PubMed]
- Arnold FL, Fukunaga S, Kusama M, et al. Assessment of factors associated with dose differences between Japan and the United States. Clin Pharmacol Ther 2014;95:542-9. [Crossref] [PubMed]
- Arnold FL, Kusama M, Ono S. Exploring differences in drug doses between Japan and Western countries. Clin Pharmacol Ther 2010;87:714-20. [Crossref] [PubMed]
- Maeda H, Kurokawa T. Differences in maximum tolerated doses and approval doses of molecularly targeted oncology drug between Japan and Western countries. Invest New Drugs 2014;32:661-9. [Crossref] [PubMed]



