
Challenges of the phase I drug development in non-small cell lung cancer
Introduction
Both targeted therapy and immunotherapy have a significant progress in past decade in non-small cell lung cancer (NSCLC). Osimertinib, the third-generation epidermal growth factor receptor (EGFR) tyrosine kinas inhibitor (TKI), is effective in EGFR-mutant NSCLC patients who had been treated with the prior-generation EGFR TKIs (e.g., gefitinib, erlotinib) and whose tumors harbor the EGFR T790M mutation as the acquired resistance (1). Osimertinib also outperforms gefitinib or erlotinib in the first-line setting (2). In ALK fusion-positive NSCLC, the second-generation ALK inhibitors are better than crizotinib in the first-line setting [e.g., alectinib (3), brigatinib (4)]. Lorlatinib, the third-generation ALK inhibitor, is useful after resistance to other ALK inhibitors (5). Immunotherapy has fundamentally changed the treatment landscape for many patients with NSCLC. An anti-programmed death receptor-1 (PD-1) and its ligand (PD-L1) antibody alone [e.g., pembrolizumab (6)] or in combination [e.g., pembrolizumab (7,8), atezolizumab (9)] with chemotherapy is now the preferred first-line option in patients with non-genomic driven NSCLC. Many targeted therapies (Table 1) and immunotherapies are currently in clinical development in NSCLC.
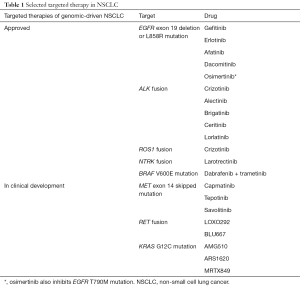
Full table
The success of the recent targeted therapy [e.g., osimertinib (10)] and immunotherapy [e.g., pembrolizumab (11)] makes some people believe that the classical concepts of oncology phase I trials, e.g., dose-limiting toxicity (DLT), maximum tolerated dose (MTD), dose-response/toxicity relationship, pharmacodynamics (PD), pharmacokinetics (PK), are no longer relevant. For example, there was neither DLT nor MTD in the first-in-human phase I trial of osimertinib. Dose-response relationship was also not demonstrated among the five doses (20, 40, 80, 160, and 240 mg/day) being tested (10). As for immunotherapy, there was no DLT in the first-in-human phase I trial of pembrolizumab. The study only reached the maximum administered dose but not MTD. Preliminary antitumor response was quickly observed in malignant melanoma and NSCLC. This phase I trial was then seamlessly transitioned to multiple expansion cohorts to define the efficacy and the optimal dose/schedule (11).
Do we have to abandon the “classical” concepts (e.g., MTD, DLT, PD, PK) to conduct phase I trials of new targeted or immunotherapies in NSCLC? In this review, I will discuss several important dimensions to argue against giving up the “classical” concepts of oncology phase I trials in NSCLC.
Defining the dose-limiting or treatment-related toxicities in the phase I trials
In the current era of NSCLC drug development, people who believe that DLT is no longer relevant usually use osimertinib as an example. Osimertinib is a drug designed and engineered driven by strong preclinical rationale, e.g., EGFR T790M mutation as the major acquired resistance to the prior-generation EGFR TKIs. Osimertinib is an inhibitor against EGFR exon 19 deletion/L858R mutation and against EGFR T790M mutation, but not against EGFR wild type (12). This design theoretically creates a wide therapeutic window. Therefore, there was neither DLT nor maximum tolerated dose (MTD) of osimertinib in the first-in-human phase I trial. The preliminary antitumor responses were observed across all five dose levels (20, 40, 80, 160, and 240 mg/day) tested (10).
Other examples to demonstrate the irrelevance of DLTs are anti-PD-1/PD-L1 antibodies. There was only one patient [with thymoma (13)] experienced a DLT (myasthenia gravis) among first-in-human phase I trials of approved anti-PD-1 [nivolumab (14), pembrolizumab (11), cemiplimab (15)]/PD-L1 [atezolizumab (16), avelumab (13), durvalumab (17)]. We have to be careful in interpreting this result, as monoclonal antibodies with their antigen-antibody binding specificity traditionally rarely lead to off-target drug-related adverse events/off-target DLTs. There is no dose-response/toxicity relationship in monoclonal antibodies (18). Anti-PD-1/PD-L1 antibodies are the mainstay of immunotherapy in NSCLC nowadays. But this class of compounds will not be the only immunotherapy in NSCLC in the future. It is better to define immunotherapy in NSCLC into two categories, the biologics [approved: anti-PD-1/PD-L1 antibodies; in development: e.g., anti-lymphocyte-activation protein 3 (LAG3) antagonistic antibodies, anti-TIM3 antagonistic antibodies, anti-OX40 agonistic antibodies, anti-4-1BB agonistic antibodies, anti- glucocorticoid-induced TNFR family related gene (GITR) agonistic antibodies] and the small molecules [in development: e.g., colony stimulating factor 1 receptor (CSF1R) inhibitors, transforming growth factor (TGF)-beta inhibitors, A2aR inhibitors]. For antibodies, “treatment limiting” (sometimes beyond cycle 1) immune-related toxicities are more appropriate DLTs than ‘traditional’ DLTs (19), as some severe/life threatening immune-related adverse events (e.g., gastrointestinal, endocrine, hepatic, pulmonary) could occur late in the whole treatment course and doses of immunotherapy are often held rather than reduced when severe/life threatening adverse events do occur (20). On the contrary, dose limiting “immune-related” toxicities are still an important component to be clearly define for the small molecule immune modulators in the first-in-human phase I trials in NSCLC.
Defining the optimal biologic or immunology doses in the phase I trials
The assumption behind the MTD is that there is a dose-response of dose-toxicity relationship. As previously mentioned, the dose-response relationship does not exist in anti-PD-1/PD-L1 antibodies (11,13-17). To push the dose of cancer immunotherapy to the highest tolerable (or administered) needs to reconsider. The optimal biologic or immunologic doses for targeted or immunotherapies, respectively, needs to be conceptualize and individualized target by target. Research in this area is highly encouraged.
Pharmacodynamics is more critical than before in the phase I trials
One of the notable examples is the phase I trials of the first-generation EGFR TKIs, gefitinib versus erlotinib. The recommended phase II dose of the first-in-human phase I trial of erlotinib is 150 mg daily, which is the MTD (21). The recommended phase II doses of 4 first-in-human phase I trials of gefitinib are 250 or 500 mg daily (22-25). The latter is the MTD and the former based on the minimal dose of normal skin (as surrogate tissue) EGFR and downstream signaling target inhibition (pharmacodynamics) (26). The 250 mg daily was chosen as the dose for the registration phase III trial after two randomized phase II trials showed equal efficacy but different toxicities in unselected NSCLC patients (27,28). This mostly explains why patients who take gefitinib usually experience less adverse events compared with those who take erlotinib (29).
The importance of pharmacodynamics in phase I trials in NSCLC has been well illustrated in the above examples. More and more phase I trials of targeted therapies and immunotherapies in NSCLC nowadays require serial (pre-treatment and on-treatment) not only surrogate tissues [e.g., normal skin for EGFR signaling, normal skin follicles for smoothened (SMO) signaling] but also tumor tissues. This is doable and does not confer too much additional risk to patients (30).
The use of adaptive phase 1 protocol
It is not uncommon to observe the preliminary antitumor activities in the phase I trials nowadays. The cohort expansion not only is a confirmatory stage of the recommended phase II dose (more adverse event and PK collection in more patients) but also serves other purposes such as evaluation of efficacy. The sample sizes of the cohort expansion should be justified with respect to their primary aim (dose-seeking based on DLTs, ineffectiveness, or target modulation) and include interim analyses to allow for early stopping.
Conclusions
I discuss the challenges in NSCLC phase I trials, such as more precise dose determinations using statistical modelling; improved selection of patients based on genetic or molecular biomarkers; pharmacokinetic and pharmacodynamic analyses; and the early evaluation of efficacy—in addition to safety. Accelerated approval pathways in major industrialized countries that can accelerate drug development require demonstration of efficacy in early phase trials. The application of molecular tumor profiling for matched therapy is increasingly seen in phase I trials. Finally, the shift towards multi-institutional trials and centralized study management results in consequent implications for institutions and investigators.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Patnaik A, Kang SP, Rasco D, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res 2015;21:4286-93. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Heery CR, O'Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017;18:587-98. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Migden MR, Rischin D, Schmults CD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med 2018;379:341-51. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol 2016;34:3119-25. [Crossref] [PubMed]
- Tosi D, Laghzali Y, Vinches M, et al. Clinical development strategies and outcomes in first-in-human trials of monoclonal antibodies. J Clin Oncol 2015;33:2158-65. [Crossref] [PubMed]
- Postel-Vinay S, Collette L, Paoletti X, et al. Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents--dose-Limiting Toxicity and Toxicity Assessment Recommendation Group for Early Trials of Targeted therapies, an European Organisation for Research and Treatment of Cancer-led study. Eur J Cancer 2014;50:2040-9. [Crossref] [PubMed]
- Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785-92. [Crossref] [PubMed]
- Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 2001;19:3267-79. [Crossref] [PubMed]
- Ranson M, Hammond LA, Ferry D, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol 2002;20:2240-50. [Crossref] [PubMed]
- Herbst RS, Maddox AM, Rothenberg ML, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol 2002;20:3815-25. [Crossref] [PubMed]
- Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol 2002;20:4292-302. [Crossref] [PubMed]
- Nakagawa K, Tamura T, Negoro S, et al. Phase I pharmacokinetic trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib ('Iressa', ZD1839) in Japanese patients with solid malignant tumors. Ann Oncol 2003;14:922-30. [Crossref] [PubMed]
- Albanell J, Rojo F, Averbuch S, et al. Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol 2002;20:110-24. [Crossref] [PubMed]
- Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290:2149-58. [Crossref] [PubMed]
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) J Clin Oncol 2003;21:2237-46. [corrected]. [Crossref] [PubMed]
- Urata Y, Katakami N, Morita S, et al. Randomized Phase III Study Comparing Gefitinib With Erlotinib in Patients With Previously Treated Advanced Lung Adenocarcinoma: WJOG 5108L. J Clin Oncol 2016;34:3248-57. [Crossref] [PubMed]
- Liao BC, Bai YY, Lee JH, et al. Outcomes of research biopsies in clinical trials of EGFR mutation-positive non-small cell lung cancer patients pretreated with EGFR-tyrosine kinase inhibitors. J Formos Med Assoc 2018;117:326-31. [Crossref] [PubMed]


