
Incidental high-grade dysplasia of the cystic duct margin: case report and literature review
Introduction
Cholecystectomy is one of the most frequent specimens in a surgical pathology practice; in the USA, 750,000 laparoscopic cholecystectomies are performed annually (1,2). A variety of incidental pathology findings can be noted, ranging from benign to premalignant and malignant lesions (3). The incidental finding of gallbladder carcinoma is an uncommon but grave event, encountered in an estimated 0.3–2.0% of cholecystectomy specimens (1,3). Invasive carcinomas frequently arise in a background of high-grade dysplasia (HGD), and the finding of HGD warrants a careful search for an underlying invasive malignancy. Isolated HGD with no invasive counterpart is a rare finding, described in the literature with no prospective studies on the long-term outcome. When detected, simple cholecystectomy is considered sufficient treatment (4). Scarce data exists on the proper management of isolated HGD at the cystic duct margin; only limited retrospective case series have investigated the outcome of these patients (1,5). We report the case of a 44-year-old patient who had incidental isolated HGD of the gallbladder involving the cystic duct and its margin. This report reviews the literature on this subject and its management.
Case presentation
A previously healthy 44-year-old man presented with a history of recurrent colicky right upper quadrant pain. Ultrasound sonography showed cholelithiasis with no signs of cholecystitis. There was no relevant past medical or surgical history and the patient did not have any history of familial neoplasia. Eventually, the patient underwent laparoscopic cholecystectomy for suspected symptomatic gallstones. Intra-operatively, the gallbladder was found fibrotic. Serosal inflammation with multiple adhesions of the gallbladder to the duodenum were described by the surgeon. The post-operative course was smooth and the patient was discharged home on day 1 post-operatively.
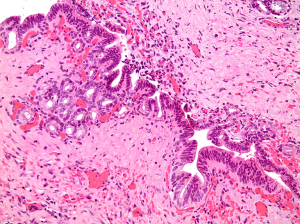
Gross examination of the pathology specimen showed a 12 cm gallbladder focally covered by fibrous adhesions. Three large gallstones had been removed by the surgeon. The wall measured up to 0.8 cm in thickness. The mucosa appeared flat. There were no discrete lesions or masses. Three sections, including the cystic duct margin, were submitted for histopathologic evaluation. Microscopic examination revealed several foci of HGD, mostly within the cystic duct, with extension to the cystic duct margin. This was exemplified by short papillary projections and fused small glands lined by atypical cuboidal to columnar cells showing loss of polarity, pseudostratification and lack of surface maturation. The cells showed nuclear enlargement and irregularity, high nuclear/cytoplasmic ratio, fine nuclear chromatin and prominent nucleoli (Figure 1). The rest of the gallbladder showed prominent chronic fibrosing inflammation with thickening of the wall and focal acute inflammation. No invasive malignancy was seen. Immunohistochemical staining for p53 revealed patchy nuclear staining of the dysplastic nuclei. This created a subtle differential gradient of staining between the benign non-immunoreactive biliary epithelium and the areas with dysplasia. Weak, non-specific staining was seen in the reactive stromal cells.

After a multidisciplinary discussion on this unexpected finding, the patient was evaluated with MRCP which showed central intra-hepatic biliary ductal dilation with tram-track appearance, possibly post-operative in nature, with no mass lesions.
Despite the absence of any recognizable residual disease, the patient underwent laparoscopic cystic bile duct exploration and excision of a 1.2 cm cystic duct stump four months after the initial cholecystectomy. An intra-operative cholangiogram through the common bile duct showed no biliary anomalies. Histologically, the additionally resected cystic duct stump demonstrated mild chronic inflammation and fibrosis. Reactive epithelial changes were seen but dysplasia was not identified (Figure 2). The patient did well postoperatively and was discharged home on day 1 post-operatively.
A MRCP done one year later showed no intrahepatic or extrahepatic biliary ductal dilatation. The previously described central dilatation was resolved. At the time of writing of this article, twenty months following the initial diagnosis of HGD, the patient remains free from recurrence.
Discussion
Incidence and presentation
The pathogenesis and incidence of gallbladder carcinoma shows geographic variation; it is most common in Chile and India (age-standardized rates from 3.9 to 8.6/100,000), while relatively rare in Europe and Northern America (6-8). In one United Kingdom study of 4027 routinely sampled gallbladders, incidental invasive carcinoma was encountered in 6 cases (rate of 0.15%), and carcinoma in situ in 1 (0.02%) (2). Of note, HGD involved the resected surgical margins in up to 9–13% of gallbladders with invasive carcinoma (1). The prevalence of isolated gallbladder dysplasia has also been variably reported. In a large study of 16,611 gallbladder resections, Renshaw et al. found 90 cases (0.05%) with dysplasia; 9 with HGD and 81 with low-grade dysplasia (3). HGD of the cystic duct is rare and its true incidence remains unknown (1,9). In a retrospective review of the archives of a reference cancer center, Bickenbach et al. found only seven cases of cystic duct HGD. Further imaging and surgical exploratory work-up detected node-positive adenocarcinoma of the cystic duct in one of five patients with available follow-up (1).
Unlike adenomas and papillary lesions, dysplasias of the gallbladder are flat lesions that do not form grossly identifiable masses or lesions (3). They can, however, result in mural thickening, as it appears to be the case in this report, and gallbladder wall thickening should prompt careful gross examination (8,10).
Risk factors associated with the development of gallbladder carcinoma correspond to events causing recurrent or continuous epithelial injury and include cholelithiasis, chronic cholecystitis, chronic infection, primary sclerosing cholangitis and abnormal choledochopancreatic duct junction (4,11). Certain ethnicities, Indian descent in particular, are factors to consider (12). Obesity has also been shown to increase the risk by 15–66% (12). Risk stratification based on these factors can potentially allow us to discern the category of patients for whom a lower threshold for further investigation and intervention would be cost-effective. Of the stated factors, our patient had cholelithiasis as evidenced by the preoperative sonographic evaluation and reported by the surgeon. The long-standing chronic inflammation that accompanies gallstone disease has been incriminated as a risk factor for developing atypia, dysplasia and carcinoma (13), with increased risk in porcelain gallbladders (3,12). Micro-lithiasis appear to be more frequently associated with the finding of metaplastic and dysplastic changes as they tend to remain subclinical for prolonged periods of time when compared to macro-lithiasis, causing more significant impact on the biliary epithelium (10).
Carcinogenesis
P53 mutation, seen in preneoplastic lesions of various cancers, is one of the earliest events in gallbladder carcinogenesis, with mutation/overexpression and/or loss of heterozygosity shown to be present in more than 50% of gallbladder carcinomas (14). P53 mutation has also been linked with gallstone disease and has been detected in the normal and dysplastic epithelium obtained from gallbladders with gallstones (7,13). Chronic epithelial injury due to cholelithiasis results in an inflammatory cascade that is postulated to trigger the overexpression and stabilization of the p53 protein (13). In our case, strong p53 staining was restricted to dysplastic nuclei, hinting at possible early manifestation of neoplastic potential. Unequivocal p53 positivity, with continuous strips of dysplastic cells showing strong nuclear staining, was not seen. As such, though p53 might prove to be a helpful adjunct in some instances, the diagnosis of dysplasia remains morphologic and primarily based on hematoxylin and eosin staining.
Following the Fearon-Vogelstein model for colorectal tumorigenesis, Barreto et al. proposed a comprehensive sequential model of gallbladder carcinogenesis from normal mucosa to invasive malignancy (7,15). The authors highlight the dysplasia-carcinoma, as well as the metaplasia-dysplasia-carcinoma sequences, as the dominant pathways for the development of gallbladder carcinomas. Roa et al. found that metaplasia, low and HGD were noted in the vicinity of invasive carcinoma in 66%, 81.3%, and 69% of cases, respectively. The adenoma-carcinoma pathway represents a secondary, minor cascade of tumorigenic events in the gallbladder (16). The similar carcinogenic mutational profile seen in dysplastic and malignant gallbladder mucosa serves as compelling support for the preneoplastic role of the former, with p53, p16, DCC, and APC gene mutations frequently observed in both (13,17). A 10-year long timeframe for the dysplasia-carcinoma pathway to fully develop has been suggested (16).
Sampling of gallbladders with HGD
The American Hepato-Pancreato-Biliary Association (AHPBA) recommends that the finding of HGD should prompt the sampling of the entire gallbladder to rule out gallbladder carcinoma and eventually stage any associated invasive malignancy (18).
Many have questioned the validity and usefulness of this approach (4,19,20). For Renshaw et al., review of the gross specimen and the submission of up to 4 additional sections successfully identified all significant lesions, and submission of the entire gallbladder is not justified (20). This is further supported by another study by Bosch et al., where selective tissue sampling following the identification of HGD on initial slides successfully identified invasive carcinoma in 10% of HGD cases (4). Follow-up of cases with a final diagnosis of HGD after selective additional sampling did not detect any subsequent adverse prognostic events (i.e., recurrence or metastasis) (4). Selective targeted sampling such as the one advocated by Bosch et al. depends on macroscopic examination. The yield of such an approach can be limited, as many cases of gallbladder carcinoma and dysplasia do not present as a grossly identifiable lesion. In the absence of a discrete mass, we elected to be generous in submitting random sections. This supplementary subtotal sampling did not reveal any additional pathological findings.
Management and follow-up of HGD at the cystic duct margin
In the management of cases similar to the one presented in this report, Moslim et al. and Bickenbach et al. recommend that upon review of pathology, patients with isolated HGD should receive high-quality cross-sectional imaging to rule out invasive carcinoma (1,5). MRCP is suggested as the optimal imaging modality, allowing the evaluation of the cystic duct remnant, the bile duct and locoregional lymph nodes (1). The finding of a locally advanced malignant process would divert management away from additional surgical intervention and favor conservative treatment. Upon negative work-up, Moslim et al. proceeded to excise the cystic duct, which did not show any evidence of HGD on pathology. Considering the young age of the patient presented and the lack of data concerning HGD, it was decided to monitor the biliary tree for 5 years for early detection of any biliary tract lesions that may occur (5). Patients of gallbladder dysplasia appear to be prone to develop multifocal biliary neoplasia and should be closely followed up for the neoplastic potential of the pancreatobiliary tree. This, together with the common embryologic origin of these organs, demonstrates a field effect (9). The finding of HGD at the cystic duct should prompt the search of a possible synchronous biliary cancer with complementary radiological examination (5).
Should further excision of the cystic duct stump be undertaken, it is suggested by some that serial intraoperative frozen-section examination be performed to achieve a negative margin (1,2). From a surgical pathologist’s standpoint, the added value of intraoperative analysis in this context is debatable. Recent or any prior surgical manipulation in the region is likely to result in marked chronic histological changes with accompanying reactive epithelial atypia in the inflamed biliary epithelium. This poses diagnostic challenge for the pathologist and might lead to overinterpretation of the pathological changes. The histology would thus best be interpreted on permanent sections to minimize the risk of overtreatment.
Conclusions
We report a case of isolated HGD of the gallbladder involving the cystic duct. Though rare, these patients represent a challenge to the pathologist and the treating team and are to be investigated for underlying invasive gallbladder carcinoma. Though opinions differ concerning adequate sampling of a dysplastic gallbladder, it remains crucial for the surgical pathologist to routinely submit a section of the cystic duct margin; if a premalignant lesion is detected, generous sampling of the gallbladder should follow to exclude frank malignancy. Additional studies are needed to better understand the long-term risk associated with premalignant lesions of the gallbladder. Cost-effective management of these lesions and optimal patient outcome can then be accomplished.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Bickenbach KA, Shia J, Klimstra DS, et al. High-grade dysplasia of the cystic duct margin in the absence of malignancy after cholecystectomy. HPB (Oxford) 2011;13:865-8. [Crossref] [PubMed]
- Patel K, Dajani K, Iype S, et al. Incidental non-benign gallbladder histopathology after cholecystectomy in an United Kingdom population: Need for routine histological analysis? World J Gastrointest Surg 2016;8:685-92. [Crossref] [PubMed]
- Rais R, Gonzalez I, Chatterjee D. Dysplasia in Gallbladder: What Should We Do? J Gastrointest Surg 2019;23:686-9. [Crossref] [PubMed]
- Bosch DE, Yeh MM, Schmidt RA, et al. Gallbladder carcinoma and epithelial dysplasia: Appropriate sampling for histopathology. Ann Diagn Pathol 2018;37:7-11. [Crossref] [PubMed]
- Moslim MA, Tang A, Morris-Stiff G. Management of high-grade dysplasia of the cystic duct after cholecystectomy. BMJ Case Rep 2017;2017. [Crossref] [PubMed]
- Bangash M, Alvi AR, Shahzad N, et al. Factors Associated with Premalignant Epithelial Changes in Chronic Calculous Cholecystitis: A Case-Control Study. World J Surg 2018;42:1701-5. [Crossref] [PubMed]
- Barreto SG, Dutt A, Chaudhary A. A genetic model for gallbladder carcinogenesis and its dissemination. Ann Oncol 2014;25:1086-97. [Crossref] [PubMed]
- Solaini L, Sharma A, Watt J, et al. Predictive factors for incidental gallbladder dysplasia and carcinoma. J Surg Res 2014;189:17-21. [Crossref] [PubMed]
- Koshiol J, Bellolio E, Vivallo C, et al. Distribution of dysplasia and cancer in the gallbladder: an analysis from a high cancer-risk population. Hum Pathol 2018;82:87-94. [Crossref] [PubMed]
- Seretis C, Lagoudianakis E, Gemenetzis G, et al. Metaplastic changes in chronic cholecystitis: implications for early diagnosis and surgical intervention to prevent the gallbladder metaplasia-dysplasia-carcinoma sequence. J Clin Med Res 2014;6:26-9. [PubMed]
- Katabi N. Neoplasia of gallbladder and biliary epithelium. Arch Pathol Lab Med 2010;134:1621-7. [PubMed]
- Pilgrim CH, Groeschl RT, Christians KK, et al. Modern perspectives on factors predisposing to the development of gallbladder cancer. HPB (Oxford) 2013;15:839-44. [Crossref] [PubMed]
- Jain K, Mohapatra T, Das P, et al. Sequential occurrence of preneoplastic lesions and accumulation of loss of heterozygosity in patients with gallbladder stones suggest causal association with gallbladder cancer. Ann Surg 2014;260:1073-80. [Crossref] [PubMed]
- Saetta AA. K-ras, p53 mutations, and microsatellite instability (MSI) in gallbladder cancer. J Surg Oncol 2006;93:644-9. [Crossref] [PubMed]
- Kai K. Organ-specific concept and controversy for premalignant lesions and carcinogenesis of gallbladder cancer. Hepatobiliary Surg Nutr 2016;5:85-7. [PubMed]
- Roa I, de Aretxabala X, Araya JC, et al. Preneoplastic lesions in gallbladder cancer. J Surg Oncol 2006;93:615-23. [Crossref] [PubMed]
- Roa JC, Roa I, Correa P, et al. Microsatellite instability in preneoplastic and neoplastic lesions of the gallbladder. J Gastroenterol 2005;40:79-86. [Crossref] [PubMed]
- Aloia TA, Jarufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. HPB (Oxford) 2015;17:681-90. [Crossref] [PubMed]
- Hartman D, Krasinskas AM, Sasatomi E. Caveat emptor: submitting the entire gallbladder in cases of dysplasia is not justified. Am J Clin Pathol 2013;139:830. [Crossref] [PubMed]
- Renshaw AA, Gould EW. Submitting the entire gallbladder in cases of dysplasia is not justified. Am J Clin Pathol 2012;138:374-6. [Crossref] [PubMed]


