
Adjuvant therapy for resected gallbladder cancer
Introduction
Gallbladder cancer, one of the family biliary tract cancers, including intrahepatic, extrahepatic, and hilar cholangiocarcinoma. It is an uncommon and aggressive cancer with a 5-year survival rate of 5% (1). Although surgical resection is the only cure, the number of patients who can have curative surgery is small and the relapse is frequent (2). Adjuvant therapy plays a crucial role in improving survival.
Prospective data on adjuvant therapy in gallbladder cancer have been limited. In addition to its rarity, gallbladder cancer patients are often frail following major surgery, making enrolment of patients with gallbladder cancer to clinical trials difficult.
In this review, the current status of adjuvant therapy, including radiotherapy, chemotherapy with or without radiation, has been summarised and future perspectives have been discussed.
Candidates for adjuvant therapy
The primary role of adjuvant therapy is to reduce the recurrence rate by eliminating micrometastatic disease that cannot be detected with imaging. The toxicity profile must be favourable to permit effective delivery (3). Simple cholecystectomy can be used for the treatment of gallbladder cancer, and long-term survival has been observed if the tumour is within the lamina propria (4,5). However, at stage I with tumours invading the mucosal layer, the 5-year survival rate decreased rapidly to 62.5% (6). Therefore, adjuvant therapy is recommended for every tumour stage according to the National Cancer Center Network guideline (7). The effect of adjuvant therapy differs among cancer stages, including tumour and lymph node stages. A meta-analysis by Horgan et al. involving 6,712 patients with gallbladder cancer (66.2%) revealed that adjuvant therapy is associated with nonsignificant improvement (P=0.06), with no difference between tumour sites, and provided the greatest benefit for lymph node-positive patients (P<0.01) (8). Another meta-analysis by Ma et al. also showed the survival benefits of adjuvant therapy in patients with gallbladder cancer with R1 who were lymph node positive and had stage II or more cancer (9). Due to the lack of randomised controlled trials, these meta-analyses are mainly based on retrospective studies (3).
Adjuvant radiation therapy
Radiation therapy had been used as the only gallbladder cancer method available before the introduction of effective anti-cancer drugs. Its main purpose was to control the local recurrence but not distant metastasis. To the best of our knowledge, only retrospective studies have been conducted and no prospective studies are available (Table 1). These retrospective studies were conducted mainly based on the Surveillance, Epidemiology, and End Results (SEER) programme. Mojica et al. analysed 3,187 patients with adjuvant radiation therapy (17%) and demonstrated its survival benefit compared with non-radiation therapy (14 vs. 8 months) (14). Wang et al. also showed similar results using a subgroup analysis of 4,187 patients and identified adjuvant radiation therapy as well as age, sex, papillary histology and stage as prognostic factors according to the multivariate analysis (13). A survival benefit was particularly observed for the T2 or higher category, in addition to being lymph node positive. It should be considered that SEER data has no information on concurrent chemotherapy and that radiation regimens varied widely.
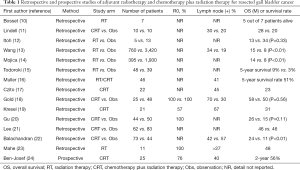
Full table
Essentially, radiation therapy is limited by micrometastasis, the majority of the first recurrence (85%) of gallbladder cancer occurring at distant sites (25). Horgan et al. showed that radiation therapy is less effective than chemotherapy plus radiation therapy or chemotherapy (8).
Adjuvant chemotherapy plus radiation therapy
Chemotherapy plus radiation therapy has been expected to yield a synergetic activity with chemotherapy and radiation therapy. In preclinical studies, some anti-cancer drugs have demonstrated radiosensitivity (26-28). Table 1 summarises the series of data of adjuvant chemotherapy plus radiation therapy.
The retrospective data available were limited and a large retrospective data had been obtained from SEER. Due to the lack of randomised trials, chemotherapy plus radiation therapy has not been established as a standard of care. Using the SEER data of 1,137 patients with chemotherapy plus radiotherapy (11%), Wang et al. showed the survival benefit of chemotherapy plus radiation therapy, especially for those with T2–4 and/or those who were lymph node positive (29). Mantripragada et al. analysed the National Cancer Database (4,775 patients) and suggested a 0.25-year survival benefit in patients with gallbladder cancer receiving chemotherapy plus radiation therapy compared with the observation group (P<0.01) (30).
Recently, one arm phase II chemotherapy plus radiotherapy study conducted by the South West Oncology Group (SWOG) evaluated gemcitabine plus capecitabine, followed by concurrent radiation (45 Gy to regional lymphatics, 54–59.4 Gy to the tumour bed) and capecitabine for cholangiocarcinoma and gallbladder cancer. In addition, the 2-year survival rate, which was the primary end-point, was 65% in biliary tract cancers and 56% in gallbladder cancer (24).
Based on the SWOG S0809 data, the American Society of Clinical Oncology (ASCO) Clinical practice guideline described that patients with gallbladder cancer with R1 resection may undergo chemotherapy plus radiation therapy (3). A randomised study should be conducted to establish the standard treatment.
Adjuvant chemotherapy
Chemotherapy has been the main treatment procedure in most malignancies. In 2010, cisplatin and gemcitabine were established as the first-line treatment for advanced biliary tract cancers, including gallbladder cancer (31). Moreover, gallbladder cancer has also been recognised as a chemo-sensitive cancer.
Table 2 shows the adjuvant chemotherapy data. As previously described, Horgan et al. published a meta-analysis that showed the survival benefit in the chemotherapy group (8) and Ma et al. also showed similar results (9). Conversely, another study that used the National Cancer Database in the U.S. did not demonstrate the survival benefit (30), and the Japanese data (n=4,700) on the adjuvant chemotherapy did not reveal any improvement in survival (32). These inconsistent data were likely to have been due to a patient heterogeneity in retrospective studies and ineffective drugs.

Full table
The first phase III trial published in 2002 by Takada et al. was a randomised study on biliary tract cancers and pancreatic cancer, including gallbladder cancer (34). Among 438 patients, 112 with gallbladder cancer were evaluated in this study, and the chemotherapy group (mitomycin and 5-FU) demonstrated a significant survival with a 5-year survival rate of 26% compared with the 14% in the observation group (P=0.04). This subgroup analysis suggested the survival benefits of adjuvant chemotherapy. The second phase III study (PRODIGE 12) examined the effects of gemcitabine and oxaliplatin in the chemotherapy group in comparison with those of the observation group (35). This study included 194 patients with gallbladder cancer (20%) with a performance status of 0–2. The primary endpoint was relapse-free survival and health-related quality of life (HRQL). Although no difference in HRQL was observed between the two groups, the chemotherapy group did not show survival benefits (median, 30.4 months in the chemotherapy arm vs. 18.5 months in the chemotherapy arm; P=0.48). In addition, a subgroup analysis showed no favourable outcomes, including the tumour site, stage and lymph node.
The BILCAP was the third randomised phase III study that evaluated adjuvant chemotherapy with capecitabine (36). This study included 447 patients with gallbladder cancer (18%), regional lymph node positive (54%) and R0 resection (62%) with a performance status of 0 and 1, and the primary endpoint was overall survival. Although no significant difference was observed in an unadjusted intent-to-treat analysis, a per-protocol analysis revealed significant results (median, 53 months in the chemotherapy arm vs. 36 months in the observation group; P=0.028) and a pre-specified intent-to-treat (ITT) analysis adjusted for nodal status, disease grade and sex also showed significant difference between the two groups. Because capecitabine can improve the overall survival, the ASCO recommends adjuvant capecitabine monotherapy for 6 months (3).
Ongoing trials and future perspective
Even in the era of systemic chemotherapy, only three randomised phase III trials on adjuvant therapy have been conducted (Table 3). The ACCTICA-1 study (NCT02170090) is currently recruiting patients with resected biliary tract cancers to examine the efficacy and safety of gemcitabine and cisplatin. According to the result of the BILCAP study, the referential arm has changed from observation to capecitabine. The ASCOT trial (UMIN000011688) compared chemotherapy group (S-1, a fluoropyrimidine drug similar to capecitabine) with the observation group. A group in China planned a phase III trial (AdBTC-1, NCT03779035) comparing gemcitabine with capecitabine and opened to accrual in 2018 (37). Besides these studies, a randomised phase II study comparing gemcitabine with capecitabine has completed the enrolment and is awaiting results.

Full table
Recently, an immune checkpoint inhibitor has been widely available for the treatment of lung cancer, melanoma, lymphoma, stomach cancer, renal cell cancer, and head and neck cancer. In resected biliary tract cancers, the cluster group had a higher expression of immune checkpoint genes as well as hypermutated genes with poor prognosis and became candidates for immune checkpoint inhibitor (38). A subgroup analysis of KEYNOTE 28 revealed that the use of pembrolizumab in patients with biliary tract cancers showed a 17%-response with manageable tolerability (39).
The progress of genomic sequence has shown genetic alteration of ERBB2 (16%), PIK3CA (14%), KRAS (11–19.2%), CDKN2A/B (19%), ARID1A (11–13%), and BAP (4–13%) in patients with gallbladder cancer (2). Although the first phase III study on advanced biliary tract cancers, using erlotinib, has reported negative results (40), many studies that have used a molecular-targeted therapy have been recruited and are expected to yield positive results. An adjuvant treatment setting is planned after the examination in advanced gallbladder cancer.
Conclusions
The current status of adjuvant therapy for the treatment of gallbladder cancer is summarized in this study. With the acceptance of chemotherapy as the active treatment by the results of the BILCAP study, chemotherapy has been the main treatment procedure in adjuvant therapy. The results of ongoing phase III studies of adjuvant chemotherapies may connect with improving outcome for gallbladder cancer. The further investigation by the well designed and randomized study will be required to examine the effect of chemotherapy plus radiation therapy.
Acknowledgments
J Bridgewater is funded in part by the ULCH/UCL Biomedical Centre.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Gourgiotis S, Kocher HM, Solaini L, et al. Gallbladder cancer. Am J Surg 2008;196:252-64. [Crossref] [PubMed]
- Bridgewater JA, Goodman KA, Kalyan A, et al. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. Am Soc Clin Oncol Educ Book 2016;35:e194-203. [Crossref] [PubMed]
- Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol 2019;37:1015-27. [Crossref] [PubMed]
- Hueman MT, Vollmer CM Jr, Pawlik TM. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol 2009;16:2101-15. [Crossref] [PubMed]
- North JH Jr, Pack MS, Hong C, et al. Prognostic factors for adenocarcinoma of the gallbladder: an analysis of 162 cases. Am Surg 1998;64:437-40. [PubMed]
- Lee AJ, Chiang YJ, Lee JE, et al. Validation of American Joint Committee on Cancer eighth staging system for gallbladder cancer and its lymphadenectomy guidelines. J Surg Res 2018;230:148-54. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Ma N, Cheng H, Qin B, et al. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer 2015;15:615. [Crossref] [PubMed]
- Bosset JF, Mantion G, Gillet M, et al. Primary carcinoma of the gallbladder. Adjuvant postoperative external irradiation. Cancer 1989;64:1843-7. [Crossref] [PubMed]
- Lindell G, Holmin T, Ewers SB, et al. Extended operation with or without intraoperative (IORT) and external (EBRT) radiotherapy for gallbladder carcinoma. Hepatogastroenterology 2003;50:310-4. [PubMed]
- Itoh H, Nishijima K, Kurosaka Y, et al. Magnitude of combination therapy of radical resection and external beam radiotherapy for patients with carcinomas of the extrahepatic bile duct and gallbladder. Dig Dis Sci 2005;50:2231-42. [Crossref] [PubMed]
- Wang SJ, Fuller CD, Kim JS, et al. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol 2008;26:2112-7. [Crossref] [PubMed]
- Mojica P, Smith D, Ellenhorn J. Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. J Surg Oncol 2007;96:8-13. [Crossref] [PubMed]
- Todoroki T, Kawamoto T, Otsuka M, et al. Benefits of combining radiotherapy with aggressive resection for stage IV gallbladder cancer. Hepatogastroenterology 1999;46:1585-91. [PubMed]
- Müller B, Sola JA, Carcamo M, et al. Adjuvant chemoradiation for resected gallbladder cancer: Treatment strategies for one of the leading causes of cancer death in Chilean women. Indian J Cancer 2013;50:184-8. [Crossref] [PubMed]
- Czito BG, Hurwitz HI, Clough RW, et al. Adjuvant external-beam radiotherapy with concurrent chemotherapy after resection of primary gallbladder carcinoma: a 23-year experience. Int J Radiat Oncol Biol Phys 2005;62:1030-4. [Crossref] [PubMed]
- Gold DG, Miller RC, Haddock MG, et al. Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys 2009;75:150-5. [Crossref] [PubMed]
- Kresl JJ, Schild SE, Henning GT, et al. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys 2002;52:167-75. [Crossref] [PubMed]
- Gu B, Qian L, Yu H, et al. Concurrent Chemoradiotherapy in Curatively Resected Gallbladder Carcinoma: A Propensity Score-Matched Analysis. Int J Radiat Oncol Biol Phys 2018;100:138-45. [Crossref] [PubMed]
- Lee HY, Kim YH, Jung GJ, et al. Prognostic factors for gallbladder cancer in the laparoscopy era. J Korean Surg Soc 2012;83:227-36. [Crossref] [PubMed]
- Balachandran P, Agarwal S, Krishnani N, et al. Predictors of long-term survival in patients with gallbladder cancer. J Gastrointest Surg 2006;10:848-54. [Crossref] [PubMed]
- Mahe M, Stampfli C, Romestaing P, et al. Primary carcinoma of the gall-bladder: potential for external radiation therapy. Radiother Oncol 1994;33:204-8. [Crossref] [PubMed]
- Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol 2015;33:2617-22. [Crossref] [PubMed]
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689-700. [Crossref] [PubMed]
- van Putten JWG, Groen HJM, Smid K, et al. End-joining deficiency and radiosensitization induced by gemcitabine. Cancer Res 2001;61:1585-91. [PubMed]
- Vallerga AK, Zarling DA, Kinsella TJ. New radiosensitizing regimens, drugs, prodrugs, and candidates. Clin Adv Hematol Oncol 2004;2:793-805. [PubMed]
- McGinn CJ, Lawrence TS. Recent advances in the use of radiosensitizing nucleosides. Semin Radiat Oncol 2001;11:270-80. [Crossref] [PubMed]
- Wang SJ, Lemieux A, Kalpathy-Cramer J, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 2011;29:4627-32. [Crossref] [PubMed]
- Mantripragada KC, Hamid F, Shafqat H, et al. Adjuvant Therapy for Resected Gallbladder Cancer: Analysis of the National Cancer Data Base. J Natl Cancer Inst 2017.109. [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: an analysis of 4,770 patients. Cancer 2007;110:572-80. [Crossref] [PubMed]
- Bergquist JR, Shah HN, Habermann EB, et al. Adjuvant systemic therapy after resection of node positive gallbladder cancer: Time for a well-designed trial? (Results of a US-national retrospective cohort study). Int J Surg 2018;52:171-9. [Crossref] [PubMed]
- Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 2002;95:1685-95. [Crossref] [PubMed]
- Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol 2019;37:658-67. [Crossref] [PubMed]
- Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73. [Crossref] [PubMed]
- Adjuvant Chemotherapy for Biliary Tract Cancer After Curative Resection (AdBTC-1). Available online: https://clinicaltrials.gov/ct2/show/NCT03779035?term=NCT03779035&rank=1
- Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003-10. [Crossref] [PubMed]
- Bang Y, Doi T, Braud F, et al. Safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: Interim results of KEYNOTE-028. Eur J Cancer 2015.S11.
- Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2012;13:181-8. [Crossref] [PubMed]


