
Gallbladder cancer: surgical management
Introduction
Gallbladder cancer is the most common biliary tract malignancy and shows striking variation in incidence across world regions. It has a propensity for early lymph node and distant metastasis (1-6). In 1958, Fortner and Pack wrote, “the 5-year survival of a patient with gallbladder cancer constitutes a medical curiosity” (7). Currently, despite improvements in detection, staging, and surgical safety, 5-year survival for operable locally advanced or node-positive gallbladder cancer remains 10–25% in Western series (3,8-13). Proper surgical selection and adequate surgery remain the cornerstones of curative treatment.
The additive benefit of adjuvant chemotherapy following surgery has been evaluated in several trials with mixed results. Gemcitabine-based chemotherapy does not appear to improve cancer outcomes (14-17). However, in a recent randomized trial, patients with resected biliary cancers (including gallbladder cancer) receiving adjuvant fluoropyrimidine-based chemotherapy had improved 2-year recurrence-free survival (HR 0.75, 95% CI: 0.58–0.98, P=0.03) and improved median overall survival in an adjusted per-protocol analysis (53 vs. 36 months, P=0.03) (13). Although this study did not meet its primary endpoint of overall survival in the intention-to-treat population nor was the treatment effect statistically significant in the gallbladder cancer subgroup, the per protocol-based results form the basis of adjuvant chemotherapy recommendations (18). The absolute survival benefit of adjuvant chemotherapy for resected gallbladder cancer remains unclear.
The most consistently reported long-term survival determinants for gallbladder cancer are (I) the ability to achieve negative surgical margins and (II) pathological stage (i.e., nodal metastases) (19-24). Surgical safety has improved, permitting safer radical resections, including hepatectomy and pancreatectomy, as well as concomitant organ resection when necessary. However, recurrence rates after surgery are as high as 60% (1,22,25-29). Due to differences in surgical approach, patient selection, and pathology review across institutions and regions, several aspects of surgical gallbladder cancer management remain controversial. For example, while all experts agree on the importance of obtaining negative surgical margins in gallbladder cancer operations, the degree of radicality required to achieve negative margins for early-stage tumors is less clear (30). Other controversial areas include the extent of surgery and timing of re-exploration for incidental gallbladder cancer and how best to select patients for surgery. In this review, we discuss the rationale for and approaches to margin-clearing surgery across different disease stages and highlight controversial areas within gallbladder cancer surgical management.
Diagnosis and staging of gallbladder cancer
The symptoms and signs of gallbladder cancer are vague, and most gallbladder cancers are diagnosed during or after cholecystectomy performed for other indications (1,21,26,31). Weight loss and jaundice, when present, generally indicate advanced disease with a low likelihood of long-term survival (32,33). Gallbladder polyps are a common finding on abdominal ultrasound, with the overwhelming majority being benign and, in general, safe to be followed with serial ultrasound exams. Larger polyps (>1 cm) are more likely to be neoplastic; however, the overall invasive cancer rate is <10% even for large polyps (34,35). When a workup for abdominal pain or biliary symptoms suggests a gallbladder mass, high-quality cross-sectional imaging (i.e., CT or MRI) is warranted to evaluate resectability, including assessing for suspicious lymphadenopathy and intrahepatic and/or peritoneal metastases. Sometimes the preliminary T stage can be ascertained by imaging studies (Table 1) (36). PET/CT and/or diagnostic laparoscopy can be useful for staging in select cases, such as patients with suspected T3 or T4 tumors (37-40). Some studies show PET/CT or laparoscopy findings will change clinical management in 15–25% of patients (38,40,41). Sensitivity of PET/CT for detecting metastatic disease at any site is 50–60%, with higher detection for nodal metastases (sensitivity 70% and specificity >90%) and lowest for peritoneal metastases (41).

Full table
Gallbladder cancers frequently metastasize to regional lymph nodes, with up to 33% of patients with T2 tumors and up to 60% of patients with T3 tumors harboring nodal metastases (9,42,43). It has long been recognized that peri-aortic and aortocaval nodes portend a similar prognosis as distant metastatic disease (44-47). Further, ‘skip’ metastases (e.g., retropancreatic nodal metastases without hepatoduodenal ligament node metastases) are found in 3–5% of patients (43,48). The number of nodal metastases, rather than the specific location, improves prognostic discrimination and has been incorporated into the most recent American Joint Committee on Cancer (AJCC) 8th edition staging scheme (36,49,50)).
Rationale for radical gallbladder resection
The gallbladder lacks a peritoneal covering on its hepatic-facing side. Instead, the boundary between the gallbladder and liver is the cystic plate, which is a continuation of Glisson’s capsule (51). For this reason, gallbladder cancers that invade the muscularis (i.e., T1b–T2) have a propensity to invade the liver. Up to 33% of patients with T2 tumors have micrometastases in the adjacent liver parenchyma (22). It is for this reason that simple cholecystectomy, performed for benign disease, is inadequate for all but the earliest-stage (i.e., T1a) gallbladder cancers. Simple cholecystectomy involves dissection between the gallbladder muscularis and the cystic plate. This approach is not optimal for patients with T2–T4 tumors as it risks leaving residual disease. Moreover, simple cholecystectomy typically does not include removal of cystic duct lymph nodes, and as a result, nodal staging is inadequate with this procedure.
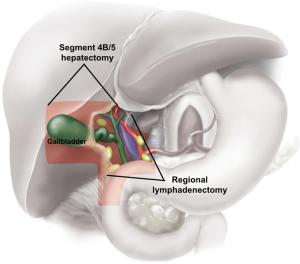
Radical (extended or margin-clearing) cholecystectomy removes the gallbladder with a margin of normal liver tissue and includes regional lymphadenectomy (Figure 1). This procedure is used to improve staging and decrease the risk of recurrence. In addition to liver resection, adequate lymphadenectomy of the porta hepatis should be performed with the goal of assessing six or more regional nodes (30,49). The degree of hepatic resection should be tailored based on the anatomic location of the primary gallbladder tumor. Experts agree that at a minimum the liver surrounding the gallbladder fossa in segments 4b/5 (Figure 1) should be resected for optimal margin clearance (30). Given the proximity of the gallbladder infundibulum to the porta hepatis and liver inflow structures (particularly the right hepatic artery, right portal vein, and right and common bile duct), tumors near the right portal structures may require more extensive hepatectomy, at times a formal right hemi-hepatectomy or right trisegmentectomy, for margin clearance. More advanced (T3–T4) tumors may involve the stomach, right colon, and/or duodenum as well as the liver. In these cases, en bloc resection of the involved organs with gastrectomy, colectomy, duodenal resection, or pancreatoduodenectomy may be required to achieve clear margins.
Primary gallbladder cancer: general approach
For suspected primary gallbladder cancer, our approach is tailored per patient to achieve adequate tumor clearance and staging while minimizing perioperative morbidity. For patients referred with a gallbladder mass, staging with high-quality cross-sectional imaging, PET/CT, and/or diagnostic laparoscopy based on tumor characteristics or imaging findings concerning for advanced-stage disease (e.g., suspicious aortocaval lymph nodes or peritoneal studding) is performed. If the staging workup is inconclusive or there is a low likelihood of cancer (e.g., a gallbladder polyp), it is reasonable to offer cholecystectomy for pathologic confirmation. Stage for stage, patients undergoing margin-clearing resection following cholecystectomy have similar long-term survival as those undergoing upfront radical resection (9,53,54). Modifications of a standard cholecystectomy, including resection of the cystic duct lymph node, are made where appropriate. If final pathology confirms T1a gallbladder cancer, no further surgical treatment is advised. For patients with T1b or higher-stage tumors who are fit for major abdominal surgery and have no evidence of inoperable disease, we offer margin-clearing surgery with regional lymphadenectomy.
Controversies in radicality: roles of extended hepatectomy and bile duct resection
A variety of approaches to ensure R0 (margin-negative) resection and adequate lymphadenectomy have been proposed. There is agreement that standard cholecystectomy does not provide adequate tumor clearance or staging information for T2–T4 tumors. However, whether to perform margin-clearing surgery for T1b tumors as well as the extent of resection required to achieve adequate clearance and staging for T2 tumors are controversial. George Pack was the first proponent of right hepatectomy for definitive gallbladder cancer surgery in the US; in his view, “the actual and potential area of spread can most adequately be removed by total right hepatic lobectomy” (7). Since Pack’s work in the 1950s, there has been persistent variation and debate regarding the surgical extent for gallbladder cancer, especially regarding the extent of hepatectomy, lymphadenectomy, routine bile duct resection (BDR), and routine port site resection (in the case of the previously explored patient).
There are no randomized trials comparing more vs. less extensive surgery for gallbladder cancer. However, there has been a growing trend away from major hepatectomy procedures and extensive resections in the absence of clinically involved structures. There is now emerging consensus that routine major liver resection is not indicated, and that view has been solidified in a recent multi-organizational joint consensus statement (30). Based on available data, we recommend (I) liver resection tailored to achieve negative margins for T1b-T4 tumors, (II) retrieving at least four, ideally at least six, lymph nodes in patients without clinically involved nodes for staging, and (III) BDR for involved cystic duct/common bile duct. We advise against major hepatectomy unless major inflow or outflow structures are involved, and we advise against routine BDR or port-site resections if those structures are not clinically involved.
Hepatectomy, bile duct resection, and extended organ resections
Between 10–25% of patients undergoing curative-intent surgery for gallbladder cancer will require major (>3 segment) hepatectomy, and up to 3% will undergo pancreaticoduodenectomy (1,12,29,55). Historically, Western series reported perioperative mortality rates as high as 20% for gallbladder cancer operations requiring major hepatectomy (56). While hepatectomy and pancreatectomy safety have improved in general, modern series show perioperative mortality remains 1–5% for patients undergoing hepatectomy or pancreatectomy for gallbladder cancer (8,9,55). While morbidity and mortality for such procedures are well-described, their value in cancer control is less well understood given the lack of prospective data assessing the influence of more extensive surgery on survival.
To address this knowledge gap, D’Angelica et al. reported their experience with 104 gallbladder cancer patients who underwent surgery from 1990–2002 (55). Nearly two-thirds of patients undergoing major hepatectomy (15% of all patients) in this series had ‘empiric’ resections, in other words, major hepatectomy in the absence of clinically involved structures mandating major hepatectomy for clearance. Patients subjected to hepatectomy for any reason had similar survival but twice the complication rate as patients undergoing less extensive liver resections (41% vs. 19%, P<0.01). All postoperative mortality (5%) occurred in patients who had major hepatectomy and BDR.
Similarly, Araida et al. showed that patients with T2 or T3 primary tumors had similar long-term outcomes regardless of the extent of hepatectomy as long as a clear margin was achieved and lymphadenectomy was performed (57). However, patients undergoing major hepatectomy had twice the postoperative complication rate as those undergoing more limited resection of the liver adjacent to the gallbladder fossa (i.e., segments 4b/5) (22% vs. 10%). In addition, a large population-based registry study from Japan also found no survival difference between radical margin-clearing gallbladder cancer resections (i.e., involving major hepatectomy or pancreatectomy) vs. more limited margin-clearing resections (partial hepatectomy) when accounting for cancer stage (20). For patients with stage II or III disease, there were no differences in R0 resection rates between 524 patients undergoing limited margin-clearing resections of the liver and/or common bile duct and the 132 undergoing radical margin-clearing resections (85–95% in each group). There was an association between radical resection and decreased 5-year survival for patients with stage III disease (39% for radical resection vs. 51% for limited resections, P<0.01). Together, these data suggest routine radical hepatectomy is unwarranted as it increases morbidity without a survival benefit and form the basis of expert guidelines advising against routine major hepatectomy (30).
Some surgeons advocated routine BDR to facilitate lymph node clearance (58). However, several studies have shown similar lymph node yield regardless of whether BDR is performed (42,49,59). Operations with BDR entail higher operative morbidity and mortality (55,59). In the D’Angelica et al. series, patients subjected to BDR for any reason had similar survival but twice the complication rate as patients without BDR (33% vs. 13%, P=0.03). Most experts, therefore, eschew routine BDR (30,60). A positive cystic duct stump margin is predictive for common bile duct involvement (42). As a result, most experts recommend selective BDR only for patients with positive cystic duct stumps and/or clinically suspicious common bile duct involvement (30).
Lymphadenectomy extent
Five-year survival for patients with lymph node metastases is 10–25% in most Western series (11,12,19,49,61). These rates are lower than those reported in Japanese and Korean series, and the reasons underlying this survival difference are currently unknown (20,44,46,62,63). Given the prognostic significance of lymph node metastases, lymphadenectomy is valuable for staging. Several groups have shown that nodal metastases are more commonly encountered when at least three lymph nodes are retrieved, while more recent data suggest four nodes may be adequate for staging (49,64,65). Furthermore, patients staged as N0 based on the retrieval of at least six lymph nodes showed improved 5-year disease-specific survival vs. those staged as N0 based on fewer nodes (72% vs. 45%, P<0.01) (49,66). These data highlight the importance of retrieving between four and seven nodes, and ideally at least six nodes, for adequate staging. They also highlight the difficulty in obtaining an adequate number of nodes. In Western series, the number of patients undergoing ‘inadequate’ lymphadenectomy (i.e., fewer than six nodes) ranges from 79–95%, whereas in a large Korean series, the proportion of patients with fewer than three nodes retrieved is 7% (12,49,63,65). The reasons underlying regional differences in lymphadenectomy yields are likely complex and beyond the scope of this review.
While the prognostic importance of lymph node metastases is well-established, the therapeutic advantage of lymphadenectomy is unclear. Among patients with nodal metastases, both the number and ratio of positive to normal nodes have prognostic utility across centers and in diverse patient populations (43,50,62,64,66,67). However, there are no randomized trials comparing outcomes for more vs. less extensive lymphadenectomy in this disease. As such, the primary roles of lymphadenectomy are for staging and guiding the use of adjuvant chemotherapy. Several groups have shown that survival rates for patients with peri-aortic lymph node involvement are similar to those with distant metastatic disease (45,62). Careful consideration should be given to assessing distant lymph nodes prior to embarking on major resection. Based on available data, we recommend routine portal lymphadenectomy with at least six nodes retrieved to ensure adequate staging. However, there are no data that support a therapeutic role for lymphadenectomy.
Surgical extent for T1–T2 cancers
T1 tumors generally have an excellent 5-year prognosis, with most series reporting survival rates of 75% or higher for patients with T1 tumors selected for surgery (20,27,31,68,69). As opposed to T1a tumors, T1b tumors are more likely to be associated with lymph node metastases and decreased 5-year survival (69-71). Some surgeons report an association between margin-clearing surgery and lymphadenectomy and improved survival for patients with T1b tumors (70,72), but others report long-term outcomes equivalent to simple cholecystectomy (68,73). Accordingly, the value of hepatic resection and lymphadenectomy remains debated for patients with T1b tumors. Most experts agree that simple cholecystectomy is sufficient for T1a tumors as the likelihood of residual disease or lymph node metastases is exceedingly small (<5%) following simple cholecystectomy (30,69). Many advocate for margin-clearing surgery and lymphadenectomy for T1b tumors given the higher rates of lymph node metastases, especially in young patients, as long as the patient is fit for surgery (30,72). We generally advise margin-clearing surgery with lymphadenectomy for young and/or fit patients.
While the debate for T1 tumors centers around simple cholecystectomy, most experts agree that more extensive surgery is required for T2 tumors. The value of hepatectomy and lymphadenectomy for T2 tumors has been reported in several studies comparing survival differences between patients who underwent such procedures to those who refused further surgery after a T2 gallbladder cancer was encountered at a previous operation (9,53,74). In all studies, patients selected for margin-clearing surgery displayed longer median and 5-year survival than those undergoing simple cholecystectomy. For example, Fong et al. showed margin-clearing or more radical resections were associated with significantly higher 5-year survival compared to cholecystectomy alone (61% vs. 19%, P<0.05) (9).
The extent of surgery required for T2 tumors may also be linked to whether the tumor originates from the hepatic or the peritoneal side of the gallbladder (22,75). Shindoh et al. assessed surgical radicality across four institutions (in Chile, Japan, Italy, and the US) focusing on survival differences according to primary tumor location (22). This study found higher rates of hepatic residual disease (33% vs. 6%, P<0.001) when patients’ primary tumors were on the hepatic side as opposed to the peritoneal side. Interestingly, patients with peritoneal-sided tumors selected for radical re-resection (margin-clearing hepatic resection and lymphadenectomy) showed increased 5-year survival rates compared to those without re-resection (76% vs. 50%, P<0.01). In contrast, hepatic-sided T2 tumors demonstrated decreased 5-year survival compared to peritoneal sided-tumors and there were no differences observed between patients selected for margin-clearing resection vs. those undergoing simple cholecystectomy alone (48% vs. 29%, P=0.19). However, other groups have not found similar associations between primary tumor location and recurrence (28). Furthermore, the biological basis for a survival difference based on T2 tumor origination site is unclear. We postulate that hepatic-sided tumors may have a greater propensity for early spread, but future studies are required to understand the reasons for the observed survival differences and whether hepatic-sided primary tumors require larger margins (more extensive liver resection) than peritoneal-sided tumors for clearance. In any case, tumor sidedness should be considered when planning the margin of adjacent liver that should be resected to ensure a clear margin is obtained.
Summary: primary gallbladder cancer
Studies to date do not support major hepatectomy or BDR in the absence of clinically suspicious disease. It is our practice to tailor surgical treatment based on the tumor stage, location (e.g., hepatic vs. peritoneal surface vs. proximity to major inflow structures), and involvement of the cystic duct stump. Removal of the gallbladder with an en bloc margin of normal tissue guides the extent of liver resection required. This is most often simple cholecystectomy for in situ cancer or T1a disease. For higher-stage tumors, we prefer, at a minimum, cholecystectomy with en bloc non-anatomic liver resection (segments 4b/5) to achieve adequate clearance. In the absence of distant disease, when the tumor involves portal structures, cholecystectomy with en bloc major liver resection is indicated for margin clearance. In addition, staging portal lymphadenectomy with the goal of retrieving six lymph nodes should be performed for all cases. If the tumor is associated with the cystic duct margin or if the common bile duct is directly involved, excision of the common bile duct (BDR) with reconstruction may also be indicated.
Controversies in incidental gallbladder cancer surgical management
Because cholecystectomy is among the most commonly performed general surgery procedures, the majority of gallbladder cancers in the US are diagnosed during or after cholecystectomy performed for other indications (1,21,26,31). Between 0.25–0.7% of cholecystectomy specimens will reveal gallbladder cancer (11,76,77). Managing these ‘incidental’ gallbladder cancers (IGBC) can be challenging because simple cholecystectomy risks inadequate clearance for many tumors and does not permit nodal evaluation. In most cases, except for the earliest-stage tumors, most experts advise repeat exploration for proper staging and removal of residual disease based on the assumption that this will alter the disease’s natural history (23,42,68,78-80). In general, patients with IGBC have higher 5-year survival rates than patients with primary or non-incidental cancers, perhaps owing to the lower stages (T1–T2 tumors) typically encountered in patients with incidental cancers (21,31,53,77,79). However, outcomes for IGBC patients seem to be largely driven by the ability to achieve margin-negative surgery and the presence of residual disease. Most reported experiences with IGBC show that 15–30% of patients selected for re-exploration will undergo a non-curative surgery (i.e., disclosing metastatic disease or leaving residual disease), which entails a dismal prognosis (Table 2) (21,26,27,31,42,54,71,81,82). The likelihood of successful margin-clearing surgery after previous exploration decreases in a stepwise fashion according to the T-stage of the primary tumor (57% for T2 tumors, 32% for T3, and 16% for T4) (9). In some series, the number of patients with inoperable disease after prior non-curative surgery is as high as 51% (83). Given these complexities, the optimal approach and timing for re-resection are debated.

Full table
Residual disease resection
The likelihood of encountering residual disease at re-exploration is directly related to the T-stage of the primary tumor (Table 3). Up to 60% of patients with T1b–T2 tumors harbor residual disease on re-exploration, either in the liver parenchyma adjacent to the gallbladder fossa, regional lymph nodes, or cystic duct stump (42,71). Lymph node metastases rates sharply rise once tumors extend through the muscularis (T2–T4). For patients at high risk of residual disease, performing diagnostic laparoscopy to evaluate resectability may avoid unnecessary laparotomy (40,54,84).

Full table
Most studies examining IGBC management have reported an association between re-resection and improved 5-year survival (9,31,53,74,79). For example, in a French multi-institution series re-resection was associated with a 26% survival advantage at 5 years (31). A German registry study reported a 16% survival advantage associated with re-resection in a similar setting (79). In both series, the association was most conspicuous for patients with T2 primary tumors but also applied to patients with T3 tumors. In other series, however, the association between re-resection and improved 5-year survival applied only to patients with T2 tumors (9,22,85).
There is a consistent association between the presence of residual disease and decreased 5-year survival across all primary tumor stages in IGBC (26,31,71,86). For example, Duffy et al. observed a median survival of 72 months among patients who underwent re-resection and were found to have no residual disease, compared to 19 months among patients with residual disease that was completely removed at re-resection (26). Similar findings have been reported by other groups (31). Recent work has focused on identifying patients likely to harbor residual disease, both for risk stratification and selection of high-risk patients who are optimal candidates for preoperative systemic chemotherapy or radiotherapy trials (87,88). These studies found that higher initial T stage, particularly T3 tumors, and poor tumor differentiation were associated with increased likelihood of residual disease.
Timing of margin-clearing surgery for incidental gallbladder cancer
In general, re-resection for patients who have already undergone simple cholecystectomy occurs at the discretion of the treating surgeon with consideration of several factors, including recovery from the initial cholecystectomy, completion of preoperative staging, and addressing complications/optimizing comorbidities for a major operation. Most patients undergo reoperation within 2–3 months of their initial cholecystectomy, but across series, the reintervention interval spans weeks to years (9,31,71,81,82). While some surgeons are concerned about disease progression while waiting for reoperation, there is no evidence that reoperation timing influences disease progression, the proportion of unresectable tumors, or survival for patients able to undergo margin-negative resection (21,54,83). Ethun et al. examined the association between reoperation timing and overall survival for IGBC using a multi-institution registry (82). Reoperation within 4–8 weeks was associated with improved overall survival compared to early (<4 weeks) or later (>8 weeks) reoperation. The authors cautioned that selection bias and small numbers in the early re-operation group influenced their results, and the reasons underlying these observed survival differences are unknown.
Given the difficulties in predicting unresectable residual disease and the uncertain influence of re-resection on IGBC natural history, several groups have reported their experience using a ‘test of time’ approach, either with or without chemotherapy, to allow disease biology to manifest and facilitate patient selection for margin-clearing surgery (81,89). In these studies, fewer than half of patients selected for deferred intervention underwent re-resection, mostly due to metastatic disease progression (peritoneal and/or liver metastases) or lack of response on imaging. While a minority were able to undergo margin-clearing surgery, median survival in this highly select group was >50 months. In addition, patients with chemotherapy response (regardless of selection for operation) showed a median survival of 12–13 months compared to four months for patients with progressive disease (89). Together these data indicate survival is better in those patients with favorable response to time or pre-surgical systemic treatment and able to undergo surgery. Thus, using time with or without other treatment modalities may be helpful for selecting IGBC patients for repeat surgery.
Port site resection
Up to 20% of patients with gallbladder cancer who previously underwent laparoscopic cholecystectomy will develop peritoneal metastases at laparoscopic port sites (90,91). Many surgeons routinely excise port sites at reoperation in patients with IGBC (53,78,90,92). It is unclear whether this practice is associated with improved cancer-specific survival. In fact, at least two groups have been unable to demonstrate a survival difference associated with routine port site resection for patients undergoing reoperation for gallbladder cancer (93,94). Moreover, both French and US multi-institutional studies have shown recurrence rates between 30–40% regardless of port-site resection. The additional morbidity of port-site resection includes a 4–15% incisional hernia rate (91,93). Since port-site resection has not been shown to improve survival or significantly reduce recurrence, we believe it should be reserved for patients with a high clinical suspicion of port-site disease involvement.
Summary
Incidentally identified gallbladder cancer presents some additional surgical considerations. While reoperation improves surgical staging and informs prognostication, between 25–60% of patients subject to re-exploration will have no residual disease and up to 60% will undergo a non-therapeutic operation. Patient selection for margin clearing surgery, the timing of the re-resection, the extent of surgery required and the use of multi-modality therapy need to be considered. To date the majority of studies addressing IGBC management report that outcomes following margin-clearing re-resection surgery are superior to simple cholecystectomy. The best results reported are for patients without residual disease selected for margin-clearing surgery. Whether re-resection improves outcomes for patients with residual disease is unclear. When patients have recovered from their cholecystectomy, we recommend thorough restaging to rule out progressive or metastatic disease. In the absence of disease progression, margin-clearing surgery is planned using the principles of surgical extent discussed above. Further studies are required to clarify the role of preoperative systemic therapy and/or radiation in this setting.
Conclusions
Surgery remains a fundamental part of gallbladder cancer management and is the only potentially curative modality. The goals of resection include cholecystectomy to obtain clear margins and adequate tumor staging. Whether this is achieved in a single operation or two separate operations depends on the clinical scenario. The extent of surgery required for margin clearance should be based on the anatomical location of the primary tumor and clinical suspicion of residual disease. No clear survival advantage has been demonstrated for routine or prophylactic major hepatectomy or lymphadenectomy in the absence of macroscopic residual tumor. Reoperation for IGBC is controversial. Methods to improve patient selection for therapeutic reoperation are needed, such as deferred reoperation with or without systemic chemotherapy, especially among patients with T2 or T3 tumors. Given the poor survival associated with locally advanced tumors or nodal metastases despite adequate surgery, we recommend a multi-modal approach for patients with gallbladder cancer.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Butte JM, Matsuo K, Gonen M, et al. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centers in three countries. J Am Coll Surg 2011;212:50-61. [Crossref] [PubMed]
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012;6:172-87. [Crossref] [PubMed]
- Witjes CDM, van den Akker SAW, Visser O, et al. Gallbladder cancer in the Netherlands: incidence, treatment and survival patterns since 1989. Dig Surg 2012;29:92-8. [Crossref] [PubMed]
- Are C, Ahmad H, Ravipati A, et al. Global epidemiological trends and variations in the burden of gallbladder cancer. J Surg Oncol 2017;115:580-90. [Crossref] [PubMed]
- Narayan RR, Creasy JM, Goldman DA, et al. Regional differences in gallbladder cancer pathogenesis: Insights from a multi-institutional comparison of tumor mutations. Cancer 2019;125:575-85. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Fortner JG, Pack GT. Clinical aspects of primary carcinoma of the gallbladder. AMA Arch Surg 1958;77:742-50. [Crossref] [PubMed]
- Benoist S, Panis Y, Fagniez PL. Long-term results after curative resection for carcinoma of the gallbladder. Am J Surg 1998;175:118-22. [Crossref] [PubMed]
- Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg 2000;232:557-69. [Crossref] [PubMed]
- Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? Cancer 2002;95:1685-95. [Crossref] [PubMed]
- Konstantinidis IT, Deshpande V, Genevay M, et al. Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: a single-institution experience. Arch Surg 2009;144:441-7. [Crossref] [PubMed]
- Mayo SC, Shore AD, Nathan H, et al. National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years: a population-based analysis. J Gastrointest Surg 2010;14:1578-91. [Crossref] [PubMed]
- Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73. [Crossref] [PubMed]
- Glazer ES, Liu P, Abdalla EK, et al. Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J Gastrointest Surg 2012;16:1666-71. [Crossref] [PubMed]
- Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 2018;105:192-202. [Crossref] [PubMed]
- Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol 2019;37:658-67. [Crossref] [PubMed]
- Malka D, Edeline J. Adjuvant capecitabine in biliary tract cancer: a standard option? Lancet Oncol 2019;20:606-8. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Hepatobiliary Cancers, version 2.2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- Fong Y, Wagman L, Gonen M, et al. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg 2006;243:767-71; discussion 771-4. [Crossref] [PubMed]
- Kayahara M, Nagakawa T, Nakagawara H, et al. Prognostic factors for gallbladder cancer in Japan. Ann Surg 2008;248:807-14. [Crossref] [PubMed]
- D'Hondt M, Lapointe R, Benamira Z, et al. Carcinoma of the gallbladder: patterns of presentation, prognostic factors and survival rate. An 11-year single centre experience. Eur J Surg Oncol 2013;39:548-53. [Crossref] [PubMed]
- Shindoh J, de Aretxabala X, Aloia TA, et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multicenter study. Ann Surg 2015;261:733-9. [Crossref] [PubMed]
- Ethun CG, Le N, Lopez-Aguiar AG, et al. Pathologic and prognostic implications of incidental versus nonincidental gallbladder cancer: a 10-institution study from the United States Extrahepatic Biliary Malignancy Consortium. Am Surg 2017;83:679-86. [PubMed]
- Oweira H, Mehrabi A, Giryes A, et al. External validation of the 8th American Joint Committee on Cancer staging system for gall bladder carcinoma. J Gastrointest Oncol 2018;9:1084-90. [Crossref] [PubMed]
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689-700. [Crossref] [PubMed]
- Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485-9. [Crossref] [PubMed]
- Barreto SG, Pawar S, Shah S, et al. Patterns of failure and determinants of outcomes following radical re-resection for incidental gallbladder cancer. World J Surg 2014;38:484-9. [Crossref] [PubMed]
- Jung W, Jang JY, Kang MJ, et al. Effects of surgical methods and tumor location on survival and recurrence patterns after curative resection in patients with T2 gallbladder cancer. Gut Liver 2016;10:140-6. [Crossref] [PubMed]
- Margonis GA, Gani F, Buettner S, et al. Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB (Oxford) 2016;18:872-8. [Crossref] [PubMed]
- Aloia TA, Jarufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. HPB (Oxford) 2015;17:681-90. [Crossref] [PubMed]
- Fuks D, Regimbeau JM, Le Treut YP, et al. Incidental gallbladder cancer by the AFC-GBC-2009 Study Group. World J Surg 2011;35:1887-97. [Crossref] [PubMed]
- Hawkins WG, DeMatteo RP, Jarnagin WR, et al. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol 2004;11:310-5. [Crossref] [PubMed]
- Regimbeau JM, Fuks D, Bachellier P, et al. Prognostic value of jaundice in patients with gallbladder cancer by the AFC-GBC-2009 study group. Eur J Surg Oncol 2011;37:505-12. [Crossref] [PubMed]
- Ito H, Hann LE, D'Angelica M, et al. Polypoid lesions of the gallbladder: diagnosis and followup. J Am Coll Surg 2009;208:570-5. [Crossref] [PubMed]
- Park JY, Hong SP, Kim YJ, et al. Long-term follow up of gallbladder polyps. J Gastroenterol Hepatol 2009;24:219-22. [Crossref] [PubMed]
- Zhu AX, Pawlik TM, Kooby DA, et al. Gallbladder. In: Amin MB, editor. AJCC Cancer Staging Manual. 8th ed. Chicago, IL: Springer, 2017.
- Corvera CU, Blumgart LH, Akhurst T, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg 2008;206:57-65. [Crossref] [PubMed]
- Butte JM, Redondo F, Waugh E, et al. The role of PET-CT in patients with incidental gallbladder cancer. HPB (Oxford) 2009;11:585-91. [Crossref] [PubMed]
- Butte JM, Gonen M, Allen PJ, et al. The role of laparoscopic staging in patients with incidental gallbladder cancer. HPB (Oxford) 2011;13:463-72. [Crossref] [PubMed]
- Davidson JT, Jin LX, Krasnick B, et al. Staging laparoscopy among three subtypes of extra-hepatic biliary malignancy: a 15-year experience from 10 institutions. J Surg Oncol 2019;119:288-94. [Crossref] [PubMed]
- Leung U, Pandit-Taskar N, Corvera CU, et al. Impact of pre-operative positron emission tomography in gallbladder cancer. HPB (Oxford) 2014;16:1023-30. [Crossref] [PubMed]
- Pawlik TM, Gleisner AL, Vigano L, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg 2007;11:1478-86; discussion 1486-7. [Crossref] [PubMed]
- Birnbaum DJ, Viganò L, Russolillo N, et al. Lymph node metastases in patients undergoing surgery for a gallbladder cancer. extension of the lymph node dissection and prognostic value of the lymph node ratio. Ann Surg Oncol 2015;22:811-8. [Crossref] [PubMed]
- Chijiiwa K, Noshiro H, Nakano K, et al. Role of surgery for gallbladder carcinoma with special reference to lymph node metastasis and stage using Western and Japanese classification systems. World J Surg 2000;24:1271-6; discussion 1277. [Crossref] [PubMed]
- Meng H, Wang X, Fong Y, et al. Outcomes of radical surgery for gallbladder cancer patients with lymphatic metastases. Jpn J Clin Oncol 2011;41:992-8. [Crossref] [PubMed]
- Kishi Y, Shimada K, Hata S, et al. Definition of T3/4 and regional lymph nodes in gallbladder cancer: which is more valid, the UICC or the Japanese Staging System? Ann Surg Oncol 2012;19:3567-73. [Crossref] [PubMed]
- Kelly KJ, Dukleska K, Kuk D, et al. Prognostic significance of the highest peripancreatic lymph node in biliary tract adenocarcinoma. Ann Surg Oncol 2014;21:979-85. [Crossref] [PubMed]
- Shirai Y, Yoshida K, Tsukada K, et al. Identification of the regional lymphatic system of the gallbladder by vital staining. Br J Surg 1992;79:659-62. [Crossref] [PubMed]
- Ito H, Ito K, D'Angelica M, et al. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg 2011;254:320-5. [Crossref] [PubMed]
- Amini N, Kim Y, Wilson A, et al. Prognostic Implications of lymph node status for patients with gallbladder cancer: a multi-institutional study. Ann Surg Oncol 2016;23:3016-23. [Crossref] [PubMed]
- Blumgart LH, Hann LE. Surgical and radiologic anatomy of the liver, biliary tract and pancreas. In: Blumgart LH, editor. Surgery of the Liver, Biliary Tract and Pancreas. 4th ed. Philadelphia, PA: Saunders, 2007:31-57.
- Qadan M, Kingham TP. Technical aspects of gallbladder cancer surgery. Surg Clin North Am 2016;96:229-45. [Crossref] [PubMed]
- Foster JM, Hoshi H, Gibbs JF, et al. Gallbladder cancer: defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol 2007;14:833-40. [Crossref] [PubMed]
- Shih SP, Schulick RD, Cameron JL, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg 2007;245:893-901. [Crossref] [PubMed]
- D'Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol 2009;16:806-16. [Crossref] [PubMed]
- Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg 1994;219:275-80. [Crossref] [PubMed]
- Araida T, Higuchi R, Hamano M, et al. Hepatic resection in 485 R0 pT2 and pT3 cases of advanced carcinoma of the gallbladder: results of a Japanese Society of Biliary Surgery survey—a multicenter study. J Hepatobiliary Pancreat Surg 2009;16:204-15. [Crossref] [PubMed]
- Shirai Y, Yoshida K, Tsukada K, et al. Radical surgery for gallbladder carcinoma. Long-term results. Ann Surg 1992;216:565-8. [Crossref] [PubMed]
- Gani F, Buettner S, Margonis GA, et al. Assessing the impact of common bile duct resection in the surgical management of gallbladder cancer. J Surg Oncol 2016;114:176-80. [Crossref] [PubMed]
- Araida T, , Higuchi R, Hamano M, et al. Should the extrahepatic bile duct be resected or preserved in R0 radical surgery for advanced gallbladder carcinoma? Results of a Japanese Society of Biliary Surgery Survey: a multicenter study. Surg Today 2009;39:770-9. [Crossref] [PubMed]
- Koh CY, Demirjian AN, Chen WP, et al. Validation of revised American Joint Committee on Cancer Staging for gallbladder cancer based on a single institution experience. Am Surg 2013;79:1045-9. [PubMed]
- Kondo S, Nimura Y, Hayakawa N, et al. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg 2000;87:418-22. [Crossref] [PubMed]
- Lee SE, Kim SW, Han HS, et al. Surgical strategy for T2 gallbladder cancer: nationwide multicenter survey in Korea. J Korean Med Sci 2018;33:e186. [Crossref] [PubMed]
- Shirai Y, Sakata J, Wakai T, et al. Assessment of lymph node status in gallbladder cancer: location, number, or ratio of positive nodes. World J Surg Oncol 2012;10:87. [Crossref] [PubMed]
- Tsilimigras DI, Hyer JM, Paredes AZ, et al. The optimal number of lymph nodes to evaluate among patients undergoing surgery for gallbladder cancer: Correlating the number of nodes removed with survival in 6531 patients. J Surg Oncol 2019;119:1099-107. [Crossref] [PubMed]
- Negi SS, Singh A, Chaudhary A. Lymph nodal involvement as prognostic factor in gallbladder cancer: location, count or ratio? J Gastrointest Surg 2011;15:1017-25. [Crossref] [PubMed]
- Liu GJ, Li XH, Chen YX, et al. Radical lymph node dissection and assessment: impact on gallbladder cancer prognosis. World J Gastroenterol 2013;19:5150. [Crossref] [PubMed]
- Aretxabala X, Ivan R, Hepp J, et al. Early gallbladder cancer: is further treatment necessary? J Surg Oncol 2009;100:589-93. [Crossref] [PubMed]
- Lee SE, Jang JY, Lim CS, et al. Systematic review on the surgical treatment for T1 gallbladder cancer. World J Gastroenterol 2011;17:174-80. [Crossref] [PubMed]
- Hari DM, Howard JH, Leung AM, et al. A 21-year analysis of stage I gallbladder carcinoma: is cholecystectomy alone adequate? HPB (Oxford) 2013;15:40-8. [Crossref] [PubMed]
- Butte JM, Kingham TP, Gonen M, et al. Residual disease predicts outcomes after definitive resection for incidental gallbladder cancer. J Am Coll Surg 2014;219:416-29. [Crossref] [PubMed]
- Goetze TO, Paolucci V. Immediate re-resection of T1 incidental gallbladder carcinomas: a survival analysis of the German Registry. Surg Endosc 2008;22:2462-5. [Crossref] [PubMed]
- Lee SE, Jang JY, Kim SW, et al. Surgical strategy for T1 gallbladder cancer: a nationwide multicenter survey in South Korea. Ann Surg Oncol 2014;21:3654-60. [Crossref] [PubMed]
- Choi SB, Han HJ, Kim CY, et al. Surgical outcomes and prognostic factors for T2 gallbladder cancer following surgical resection. J Gastrointest Surg 2010;14:668-78. [Crossref] [PubMed]
- Lee W, Jeong CY, Jang JY, et al. Do hepatic-sided tumors require more extensive resection than peritoneal-sided tumors in patients with T2 gallbladder cancer? Results of a retrospective multicenter study. Surgery 2017;162:515-24. [Crossref] [PubMed]
- Choi KS, Choi SB, Park P, et al. Clinical characteristics of incidental or unsuspected gallbladder cancers diagnosed during or after cholecystectomy: a systematic review and meta-analysis. World J Gastroenterol 2015;21:1315-23. [Crossref] [PubMed]
- Lundgren L, Muszynska C, Ros A, et al. Are incidental gallbladder cancers missed with a selective approach of gallbladder histology at cholecystectomy? World J Surg 2018;42:1092-9. [Crossref] [PubMed]
- Fong Y, Heffernan N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy: aggressive reresection is beneficial. Cancer 1998;83:423-7. [Crossref] [PubMed]
- Goetze TO, Paolucci V. Adequate extent in radical re-resection of incidental gallbladder carcinoma: analysis of the German Registry. Surg Endosc 2010;24:2156-64. [Crossref] [PubMed]
- Søreide K, Guest RV, Harrison EM, et al. Systematic review of management of incidental gallbladder cancer after cholecystectomy: management of incidental gallbladder cancer after cholecystectomy. Br J Surg 2019;106:32-45. [Crossref] [PubMed]
- Ausania F, Tsirlis T, White SA, et al. Incidental pT2‐T3 gallbladder cancer after a cholecystectomy: outcome of staging at 3 months prior to a radical resection. HPB (Oxford) 2013;15:633-7. [Crossref] [PubMed]
- Ethun CG, Postlewait LM, Le N, et al. Association of optimal time interval to re-resection for incidental gallbladder cancer with overall survival: a multi-institution analysis from the US Extrahepatic Biliary Malignancy Consortium. JAMA Surg 2017;152:143-9. [Crossref] [PubMed]
- Tsirlis T, Ausania F, White S, et al. Implications of the index cholecystectomy and timing of referral for radical resection of advanced incidental gallbladder cancer. Ann R Coll Surg Engl 2015;97:131-6. [Crossref] [PubMed]
- Agrawal S, Sonawane RN, Behari A, et al. Laparoscopic staging in gallbladder cancer. Dig Surg 2005;22:440-5. [Crossref] [PubMed]
- Goetze TO, Paolucci V. Benefits of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German Registry. Ann Surg 2008;247:104-8. [Crossref] [PubMed]
- Gil L, de Aretxabala X, Lendoire J, et al. Incidental gallbladder cancer: how residual disease affects outcome in two referral HPB centers from South America. World J Surg 2019;43:214-20. [Crossref] [PubMed]
- Creasy JM, Goldman DA, Gonen M, et al. Predicting residual disease in incidental gallbladder cancer: risk stratification for modified treatment strategies. J Gastrointest Surg 2017;21:1254-61. [Crossref] [PubMed]
- Ethun CG, Postlewait LM, Le N, et al. A novel pathology-based preoperative risk score to predict locoregional residual and distant disease and survival for incidental gallbladder cancer: a 10-institution study from the U.S. extrahepatic biliary malignancy consortium. Ann Surg Oncol 2017;24:1343-50. [Crossref] [PubMed]
- Creasy JM, Goldman DA, Dudeja V, et al. Systemic chemotherapy combined with resection for locally advanced gallbladder carcinoma: surgical and survival outcomes. J Am Coll Surg 2017;224:906-16. [Crossref] [PubMed]
- Paolucci V, Schaeff B, Schneider M, et al. Tumor seeding following laparoscopy: international survey. World J Surg 1999;23:989-95. [Crossref] [PubMed]
- Maker AV, Butte JM, Oxenberg J, et al. Is port site resection necessary in the surgical management of gallbladder cancer? Ann Surg Oncol 2012;19:409-17. [Crossref] [PubMed]
- Drouard F, Delamarre J, Capron JP. Cutaneous seeding of gallbladder cancer after laparoscopic cholecystectomy. N Engl J Med 1991;325:1316. [Crossref] [PubMed]
- Fuks D, Regimbeau JM, Pessaux P, et al. Is port-site resection necessary in the surgical management of gallbladder cancer? J Visc Surg 2013;150:277-84. [Crossref] [PubMed]
- Ethun CG, Postlewait LM, Le N, et al. Routine port-site excision in incidentally discovered gallbladder cancer is not associated with improved survival: a multi-institution analysis from the US Extrahepatic Biliary Malignancy Consortium. J Surg Oncol 2017;115:805-11. [Crossref] [PubMed]


