Targeted therapy in soft tissue sarcoma—a novel direction in therapeutics
Introduction
Soft-tissue sarcomas (STS) are rare malignant neoplasms of mesenchymal origin. In Australia, there are about 800 new STS cases reported each year, which is a 40% increase over the past 10 years from 1998 to 2008. This represents about 1% of all neoplasm cases annually and is close to occurrence rates worldwide (1). There are more than 50 different histological subtypes with varying clinical presentations, disease progressions and responses to treatment. Current treatment modalities are limited. Surgery gives the best chance of cure for localized disease. Radiotherapy is often offered adjuvantly to reduce the risk of local recurrence. The response to chemotherapy in many STS is variable, with some subtypes being resistant and others having responses of limited duration (2). Nevertheless it is still indicated in adjuvant protocols (3) as well as in the palliative setting (4). It has been reported that the median overall survival period upon commencing palliative first- and second-line chemotherapy treatment are 12 and 8 months respectively (5).
In this review we have chosen to focus upon the emerging field of highly specific targeted therapy. Definitions of targeted therapy vary, but there is general consensus that it is a form of drug therapy that inhibits a ‘target’ within one of the cellular signaling pathways which must be measurable and involved in tumourigenesis, angiogenesis, progression and metastasis in cancer cells. Targeted therapy has potential as a novel treatment modality against STS but the exact mechanisms of the drugs and the ‘cancer profiles’ of the different histological STS must be well understood in order to achieve maximum therapeutic effect (6).
Tyrosine kinase (TK) receptors and their downstream signaling cascades
The biochemistry of TK receptors and their downstream cascades are well-documented. A TK receptor can be activated by a number of ligands, including epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) (7), insulin-like growth factor (IGF) (8). Attachment of one such ligand to the respective receptor activates its TK domains which then in turn activate a group of complex signaling cascades, including the RAS/RAF/MEK/ERK, PI3K/AKT and JAK/STAT pathways. In normal cells, these pathways are tightly regulated and are important in cell survival. However, in cancer cells, there is constitutive activation of the pathways and this leads to cell proliferation, tumourigenesis and metastasis (7). In the past decade, crosstalk between these pathways has been found to cause resistance to the primary targeted therapy drug. For example, aberrant RAS activation is known to activate the PI3K/AKT/mTOR pathway which could serve as an alternative route even though the RAS/RAF/MEK/ERK pathway has been blocked. Figure 1 provides a simplified representation of the downstream cascades and the crosstalk among them (9-13).
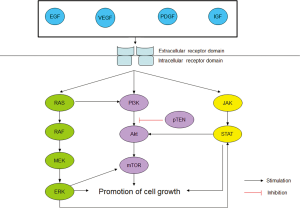
Targeted therapies for STS
Targeted therapy has now been shown to have a significant impact on the natural history of some cancers. Herceptin interferes with HER 2 signaling by targeting the HER 2 receptor in breast cancer and when used both alone and with chemotherapy leads to improved survival (14,15). Similarly the use of EGFR inhibitors in lung cancer and colon cancer has altered outcomes (16,17).This section will discuss some of the recent pre-clinical and clinical data of targeted therapy used in STS.
Tyrosine kinase Inhibitor (TKI)
TKI imatinib has great historical significance as it is the first targeted therapy against STS gastrointestinal stromal tumor (18) with significant survival benefit. In the 1980s, GIST was recognized as a separate tumor entity as scientists began to realize that it has clinical, histopathological and molecular biological features that are different from the other STS. Initially surgery was the only curable treatment option. Even then, only 50% of GIST patients without metastasis would be cured and the outlook for patient with metastasized GIST was much worse. Radiotherapy and conventional chemotherapy had no role to play in treatment at all (19). However in 1998, Hirota and colleagues discovered that the KIT oncogene, which codes for KIT receptor TK, was mutated in the majority of these patients (20). It took only another two years to produce imatinib, a small molecule oral inhibitor of KIT (19).
Imatinib is sometimes called the ‘wonder drug’ for this chemotherapy-resistant disease. Large phase II and III trials have reported 65% to 70% of GIST patients respond to imatinib, and an additional 15% to 20% achieve disease stabilization (21,22). For patients who are responsive to imatinib, the median times of progression and survival have been extended to two and five years respectively (19). Its role in a neoadjuvant setting has not yet been definitively explored, but is of interest. However, about 15% of GIST patients are resistant to imatinib (19). Many of those who responded to imatinib will have disease progression within 2 years due to acquired resistance because of a second mutation. It is unclear with current molecular studies whether this second mutation has been already present where imatinib is a selection pressure, or if it has been acquired during treatment. What is known is that this second mutation commonly occurs in KIT exon 13 or 17 (23). A number of strategies have been developed to overcome this resistance (19).
The first strategy is to lengthen the duration of imatinib treatment. In one recent adjuvant study, 400 patients who were randomized equally between one and three years of adjuvant imatinib treatment for operable GIST were compared. It was found that patients in the experimental group had longer recurrence-free survival (RFS) (5-year RFS, 65.6% vs. 47.9%, respectively) and longer overall survival (5-year survival, 92.0% vs. 81.7%) than the one year group. Imatinib was well-tolerated in both groups (24). Another strategy is to develop drugs with additional mechanisms of action to overcome resistance. For example, in a study involving 312 patients with unresectable imatinib-resistant GIST, median time to tumor progression was 27.3 weeks for those on sunitinib (which also targets PDGF and VEGF) and 6.4 weeks for those in the placebo group who had the best supportive care (25). Subsequently in an international study of another multargeted TKI, regorafenib, as third line therapy for GIST after failure of imatinib and suntinib, 199 patients were randomized. 133 receive regorafenib while 66 were in the placebo group. Median progression-free survival (PFS) for the experimental group was statistically significantly longer: 4.8 months compared to the placebo’s 0.9 months. Adverse events were mild and manageable (26). In another study of 124 patients, the efficacy of 400 mg twice daily sorafenib was investigated. 12 (10%) responded to sorafenib and 70 (57%) had disease stabilization. Like the regorafenib trial, adverse events were mild and manageable (27). The success story of imatinib in GIST serves as a good learning model for the use of targeted therapy in STS and strategies in overcoming resistance.
Another sarcoma in which imatinib has been approved for use is dermatofibrosarcoma protuberans (DFSP). DFSP is a rare dermal STS which typically carries a translocation between chromosomes 17 and 22 which produces functional PDGF B. Hence, it is only logical that imatinib, which can inhibit PDGFR, be used against DFSP. In a dual phase II study, 46% of the 24 patients evaluated had partial responses. Median time to progression was 1.7 years. Median OS time was not achieved by any patients. It concluded that imatinib is active against DFSP, although there was no statistical difference between the low dose (400 mg daily) and high dose (400 mg twice daily) (28). Another similar study in 275 patients, it also found that imatinib is active in DFSP. Secondary resistance in DFSP is unclear but is not related to PDGF B (29).
Apart from imatinib, other TKIs have also been used in other sarcoma histological subtypes. Pazopanib is a multi-targeted inhibitor of TK approved for use in renal cell carcinoma, ovarian cancer and, more recently, STS (30). In the treatment of STS, It was initially shown to have significant responses in the phase II setting in leiomyosarcoma, synovial sarcoma and other STS. Its activity in liposarcoma is less clear (31). Subsequently in a recently concluded phase III study of those responding subtypes, 369 angiogenesis inhibitor-naïve patients with metastatic STS who had failed first line chemotherapy were recruited. Patients were assigned randomly in the ratio of 2:1 (experiment:placebo). Patients in the experimental group were given pazopanib 800 mg daily. Median PFS were 4.6 months [95% confidence interval (CI): 3.7-4.8 months] and 1.6 months (0.9-1.8 months) for pazopanib and placebo respectively. OS were 12.5 and 10.7 months with pazopanib and placebo respectively. Although grade 3-4 toxicities were more common in the experimental group, they were generally well-tolerated (32). As a result of this study, STS is now also an approved indication. More importantly, pazopanib is the first new drug approved for treatment of non-GIST STS in many years. Its indication for leiomyosarcoma, synovial sarcoma and other non-GIST sarcoma has provided a new treatment option for this group of rare tumors. Further exploration of the less sensitive subtypes, such as liposarcoma, is on-going.
The signal transducer and activator of transcription 3 (STAT3) inhibitors
The STAT3 pathway is an important regulator of differentiation, proliferation, survival and angiogenesis of a normal human cell. Constitutive activation of STAT3 has been found to be involved in a number of cancers, and more recently and relevantly STS (33). The use of STAT3 inhibitors in STS is still in pre-clinical experimentation. Wang and colleagues have explored this potential. Following Western Blot data showing that 5 out of 6 STS cell lines expressed constitutively activated STAT3, they used the STAT3 inhibitor S3I-201 and showed an anti-proliferative effect on the majority of STS cell lines that harbored aberrant STAT3 (34). More pre-clinical in vivo studies are awaited.
mTOR inhibitors
The mTOR pathway tightly regulates protein synthesis and cell homeostasis. Deregulation of this pathway is strongly linked to many hematological malignancies and solid tumors. In the mTOR pathway, the activation of a TK leads to activation of PI3K. Activated PI3K induces the activation of AKT, which in turns activate mTOR. This pathway is tightly regulated, for example by pTEN which inhibits PI3K (9), Aberrant expression or activation of any of these downstream signaling proteins contributes to tumor progression (35). GISTs, for example, have a high frequency of mutation of TK receptors, while other non-GISTs have a significant deregulation of the IGFR1 pathway, both of which lead to up-regulation of the mTOR pathway (36).
mTOR inhibitors have shown clinical activity in renal carcinoma, pancreatic neuroendocrine tumors and breast carcinoma (37-42). In STS there is emerging evidence of activity. An example of a non-GIST sarcoma in which mTOR inhibition has potential is perivascular epithelioid cell tumor (PEComa). PEComas are a group of rare sarcoma that has a high frequency of the tuberous sclerosis complex. Both TSC1 and TSC2 genes commonly implicated. Recent published data has suggested that mTOR inhibition has significant activity against PEComas (43), although there are cases of resistance (44).
Ridaforolimus is a mTOR inhibitor that has been trialed for advanced bone and soft tissue sarcoma. Patients with a wide range of histological subtypes who had received a variety of chemotherapy regimens and shown a response then received ridaforolimus 12.5 mg IV over 30 minutes once daily for 5 days every 2 weeks. Efficacy assessment and restaging was done every 8 weeks until disease progression or treatment plan change. A total of 212 patients were evaluated, 61 patients (28.8%) had clinical benefit response (CBR). Median PFS and OS were 15.3 and 40 weeks, respectively. Main adverse effects were stomatitis, rash, mouth ulceration, mucosal inflammation, and fatigue but were generally well-tolerated (45). However, this trial did not distinguish the outcome of the different histological subtypes, but rather analyzed them as a whole. A subsequent phase III trial was conducted to examine ridaforolimus as maintenance therapy after standard cytotoxic chemotherapy. Patients were randomized. The experimental group had 40 mg ridaforolimus once daily 5 days each week. The primary endpoint was PFS and secondary endpoint was OS, safety and tolerability. Median OS was 93.3 weeks with ridaforolimus compared with 83.4 weeks of the placebo group [hazard ratio (HR) =0.88; 95% CI: 0.72-1.08; P=0.23]. However PFS was significantly improved (HR =0.72; 95% CI: 0.61-0.85; P=0.0001; median PFS: 17.7 vs. 14.6 weeks). Drug was again well tolerated with predictable adverse events as seen in the previous phase II trial. Although these trials have shown statistically significant changes, their clinical impact is less clear (46). Additional studies using another mTOR inhibitor, everolimus are ongoing (36).
Anti-VEGF agents
VEGF is a ligand that is responsible for inducing angiogenesis, which results in tumor blood vessel formation. In vitro experiments have shown that VEGF is an important factor in STS growth, metastasis and chemoresistance due to the close relationship between STS cells and tumor-associated endothelial cells (47).
As mentioned before, the phase III trial of pazopanib, an anti-VEGF agent, on chemotherapy-failing nonadipocytic STS was clinically active. However, resistance against it appeared to be inevitable for most patients [38]. Strategies to overcome this are to use it in combination with other targeted therapy or chemotherapy. However, this is difficult as mechanisms of resistances are unclear (48).
Bevacizumab is a human monoclonal anti-VEGF antibody that is currently used in many solid tumor malignancies. It and other VEGF inhibitors were the first systemic therapies to have any major survival impact on renal cancer (49). In addition, survival rate improvements, when combined with chemotherapy, have been described in colon cancer (50). In STS, its single-agent activity has been described against metastatic or unresectable angiosarcoma and epithelioid hemangioendotheliomas (EHE). In a phase II trial of 32 patients, investigating bevacizumab as treatment for angiosarcoma 15 mg/kg of the drug was given intravenously every 3 weeks until progression or intolerable toxicity. 48% of the patients showed stable outcome, while 15% showed partial response (51). However, since most of the patients had EHE, which can be indolent for an extended period of time, this disease stabilization may reflect natural history and not drug effect.
Type 1 IGF receptor (IGF-1R) antibody
IGF-1R is a transmembrane TK receptor that is activated by IGF under normal cell physiology, resulting in cell proliferation by angiogenesis, although the mechanism is different from TKIs as explained earlier. IGF-1R has been determined to be over expressed in a number of solid tumors. In sarcoma, IGF-1R overexpression is seen in rhabdomyosarcoma, synovial sarcoma, leiomyosarcoma, Ewing’s sarcoma (ES) and osteosarcoma. Experimental and clinical evidence shows that is associated with tumor development and progression, although the exact mechanism is yet to be determined (52).
There are a number of studies investigating the efficacy of IGF-1R antibody on ES. One of such antibody is figitumumab. In a phase I study the safety, pharmacokinetics and preliminary activity of this drug was investigated for ES. It was shown to be well tolerated with few grade 4 non-hematological toxicities and dose-limiting toxicities (53). In another phase I/II trial that investigated the efficacy of figitumumab against refractory ES, patients aged between 10 to 18 years were recruited. In the phase I part of the study, they were put into two dose-escalation cohorts (20 and 30 mg/kg intravenously every 4 weeks). The phase II portion of the study investigated the objective response rate. Like the previous study, little dose-limiting toxicities were reported for the phase I portion. In the phase II portion of the study, figitumumab as a single agent had modest anti-tumor activity, 30 mg/kg was given every 4 weeks, for a median of 2 cycles (range, 1 to 16 cycles) to 107 patients. 106 patients were suitable for evaluation, 15 and 25 had partial response and stable disease respectively. Median OS was 8.9 months. Additionally, median OS was 3.6 months for patients with a pre-treatment circulating free IGF-1 <0.65 ng/mL, while that figure was 10.4 months (P<0.001) for those with a baseline free IGF-1 ≥0.65 ng/mL. What this indicates is that it is a strong predictor of drug response (54). This supports earlier published data that insulin receptor signaling strength is a strong determinant of the efficacy of anti-IGF-1R therapies in ES (55).
R1507 is another monoclonal antibody to IGF1 receptor for recurrent or refractory Ewing sarcoma family of tumors (ESFT) examined in a phase II trial. In this study, 115 patients with recurrent or refractory ESFT aged 2 to 21 years old were recruited, 9 mg/kg/week or 27 mg/kg/3 weeks of R1507 were given to them. The drug was well-tolerated. Overall complete/partial response rate was 10%. Median duration of response and overall survival were 29 weeks (range, 12 to 94 weeks) and 7.6 months (95% CI, range, 6 to 9.7 months) respectively. It was also noted that ESFT of bone primary was more likely to respond to the drug than extra skeletal primary (56). Interestingly, in another similar phase I/II trial involving another monoclonal antibody to IGF1 cixutumumab (IMC A12) the single-agent activity of cixutumumab was limited even though it was tolerated by the patients. Serum IGF1 levels increased after the first dose of cixutumumab and the level of tumor IGF1 receptor expression did not correlate with response (57). We believe that the discrepancy between the trials could be due to several reasons. Firstly, the drugs used were different, even though the target is the same. Secondly, the recruitment criteria were different. In the first study, there was a strong emphasis on ESFT, recruiting 115 patients, while in the second study, there were only 10 ES patients in addition to another 37 solid tumor participants. The limited sample size could have caused the discrepancy. Lastly, the assessment criteria were differently. In the first study, there was an emphasis on response rate, overall survival and duration of response. In the second study, the level of serum IGF1 and expression of IGF1 receptors were used as markers of response. Despite the disappointing finding by Malempati and colleagues, they suggested that combining monoclonal antibody to IGF1 with other agents could overcome the resistance. This will be discussed below.
Met inhibitors
MET is another class of receptor TK that is activated by hepatocyte growth factor (HGF). MET is critical in sending signals that is important for cell survival and long distance migration of epithelial and myogenic precursor cells in embryogenesis. In cancer cells, this system becomes deregulated, resulting in invasion and metastasis (58). Its dysregulation has been reported in some STS (59) and has been targeted in microphthalmia transcription factor-associated (MiT) tumors such as clear cell sarcoma (CCS) and alveolar soft part sarcoma (ASPS) (60). In CCS, in vitro studies have shown that targeting MET could be a viable treatment option for the otherwise fatal disease. In a study by Davis et al. [2010], CCS cell lines were found to have a significantly higher expression of c-MET than other sarcoma cell lines. Blocking of c-MET activity with inhibitor SU11274 or neutralizing antibody to HGF AMG102 was able to significantly reduce CCS cell growth in culture. AMG102 was also shown to suppress in vivo tumor in a xenograft model (61). ASPS is another MiT sarcoma that is very rare (0.5% to 0.9% of all STS) and chemoresistant, causing it to be extremely fatal without a cure. Recently c-MET expression was found to correlate with this rare sarcoma. In a study by Hyun et al. [2010], 12 patients with ASPS were recruited to understand the genetic basis of the disease. Using immunostaining methods, 100% of the patients were TFE3 positive, a gene that activates MET expression. 75% of them were MET positive. A strong association between TFE3 and MET were found, with correlation coefficient =0.808 (P=0.02). This makes targeting MET an attractive option for both of these very aggressive MiT sarcomas (62).
In a recently concluded phase II trial that targeted MET in MiT patients, 47 patients were recruited (27 ASPS, 11 CCS, 6 translocation-associated renal cell carcinoma and 3 other types). They were given 120 mg BID and 360 mg BID in 28-day cycles after amendment to protocol. 1 CCS patient (2%) had partial response in a CCS patient (2%). Stable disease was seen in 28 patients (60%). Median PFS were 5.5 and 1.9 months for ASPS and CCS respectively. Similar to the previous studies, MET expression was found to be strongly related to these tumors. Overall although the MET inhibitors were well tolerated in these patients the efficacy was modest (60).
RANK ligand inhibitor
A recent development has been the identification of targets beyond the signaling pathways elaborated above. In some cancers, other molecules are aberrantly expressed. One example is giant cell tumor (GCT). GCT is a rare primary bone tumor with few systemic treatment options. Although often benign, some of them can be highly malignant with multiple local recurrences and metastasis. Histological studies of GCT of bone reveal that it is made up of layers of neoplastic mononuclear cells, and osteoclast-like giant cells are found distributed evenly (63). These giant cells and mononuclear cells express RANK and RANK ligand respectively. Hence, the aberrant expression of RANK ligand is responsible for the aggressive osteoclastic activity it would be a logical target as a novel systemic treatment (64).
In a phase II study, denosumab, a monoclonal antibody which inhibits RANK ligand, was administered subcutaneously 120 mg monthly every 28 days until complete resection, disease progression without clinical benefit or patient’s decision to stop. 37 patients were recruited but 2 had insufficient data for analysis. For the remaining 35 participants, 30 met tumor response criteria (86%, 95% CI: 70% to 95%) at 25 weeks. Grade 3 to 5 toxicities were reported for example, pain in the extremities (n=7), back pain (n=4) and headache (n=4). Denosumab could be the new effective systemic treatment for GCT (65). However, the exact mechanism of this drug on this disease is unknown even with histological studies. More studies are needed, but it is definitely a promising option for patients with this rare tumor.
PI3K and AKT inhibitors
Although AKT and PI3K inhibitors are in pre-clinical and clinical development and are biologically plausible targets in this group of tumors, they have yet to be examined in any detail in STS.
Combination therapy
Despite the promising theoretical potential of targeted therapy, many of these agents have limited effect when used singly. One approach to enhance the anti-tumor effects of monotherapy has been to combine them with conventional chemotherapy. These combinations have been shown to be synergistic in several studies and therefore potentially more potent in their ability to alter outcomes (66-68). This strategy has been effective in some sarcomas. This will be discussed here.
mTOR inhibitor with chemotherapy
Combination of mTOR inhibitor and combination therapy has been studied in STS in both preclinical and clinical trials. In a study by Houghton and colleagues, rapamycin was combined with cyclophosphamide, vincristine or cisplatin was examined. In vitro studies revealed that this combination was generally subadditive or additive. In in vivo studies, combination of rapamycin with cyclophosphamide or vincristine showed therapeutic enhancement, with the former more superior. However, combination of rapamycin and cisplatin produced more excessive toxicity and less therapeutic enhancement when compared to the other two combinations (69). These promising findings led to further clinical trials.
In a recent phase II study, the efficacy of sirolimus and cyclophosamide combination in advanced sarcoma was investigated. 49 patients were recruited. They were given together, sirolimus (12 mg dose on day 1 followed by 4 mg daily) and cyclophosamide (200 mg orally daily for 7 days every other week). Toxicity and 6-month PFS rates were used as assessment parameters. Grade 3 toxicities were generally well-tolerated. 10 of 47 assessable patients had PFS ≥6 months. 2 patients completed more than 12 months of treatment. 6-month PFS rate was 21% (SD: 0.06%). Median PFS and survival were 103 and 328 days respectively (70), which for such a late group was an encouraging result.
Anti-VEGF agent with chemotherapy
Anti-VEGF agent bevacizumab has been trialed in combinations with other chemotherapy drugs in soft tissue sarcoma. In 2005, D’Amato and colleagues concluded a phase II trial involving doxorubicin and bevacizumab in patients with metastatic STS. In this study, 17 suitable patients were recruited. Doxorubicin was given at 75 mg/m2 IV push followed by bevacizumab 15 mg/kg IV every 3 weeks, 2 patients showed partial responses, 11 patients showed stable disease for four cycles or more. 6 of them developed cardiac toxicity grade 2 or worse (4 grade 2, 1 grade 3 and 1 grade 4). 1 patient died of recurrent bilateral pneumothoraces, which could have been related to treatment, but had pre-existing lung lesion. This study showed that although this combination may be useful against STS, further studies should consider a lower dose in light of toxicity (71). More recently, there was a phase IB trial involving combining bevacizumab with docetaxel and gemcitabine in patients with advanced of recurrent STS. In this study, 38 suitable patients, who were chemotherapy-naïve, were recruited. Docetaxel, bevacizumab, and gemcitabine were given at 50 mg/m2, 5 mg/kg, and 1,500 mg/m2 respectively every 2 weeks after a dose-finding study for gemcitabine was conducted. Overall response rate was 31.4%, 5 had complete responses, while 6 showed partial responses. 18 had stable disease for a median of 6 months. Hematological toxicity was insignificant. However, adverse events of highest grade observed were bevacizumab-related. This trial concluded that this combination is safe and effective in STS, but the benefit of bevacizumab is uncertain (72).
Another anti-VEGF agent sorafenib has also been trialed with conventional chemotherapy, albeit with mixed outcome. They are only phase I trials. The first study involved combination of sorafenib and dacarbazine. 17 patients were with STS were recruited after failing 2 or more chemotherapy regiments and performance status of ≤2. Sorafenib was given at 400 mg twice daily while dacarbazine was given at 300 mg/m2 for 3 consecutive days every 3weeks until disease progression or intolerable toxicity. 14 patients were evaluated. 3 patients had to stop treatment early (1 died but not disease related, 2 left because of rapidly worsening condition). Of the 14 patients, 3 (21%) had partial responses, 6 (43%) had stable disease, and 5 (36%) had progressive disease. Median time of progression and overall survival were 20 weeks (range, 9-34 weeks) and 43 weeks (range, 17-65 weeks). Skin reactions (57%), fatigue (50%), neutropenia (36%) thrombocytopenia (36%) and hypertension (36%) were the main toxicities observed. 5 patients needed dose reduction for both drugs for toxicity, while 3 needed dose reduction of sorafenib (73). In another study, sorafenib was combined with ifosfamide for patients with advanced sarcoma. 12 patients were recruited. Sorafenib plus ifosfamide were administered with initial doses of 200 mg bid and 6 g/m2 respectively. Cohorts of 3-6 patients were used in a 3+3 dose escalation design. 3 dose limiting toxicity were observed (fatigue grade 4 with sorafenib 400 mg bid plus ifosfamide 6 g/m2 and encephalopathy and emesis grade 3 with sorafenib 400 mg bid plus ifosfamide 7.5 g/m2) 8 patients had stable disease for more than 12 weeks. This phase I trial concluded that sorafenib 400 mg bid plus ifosfamide 6 g/m2 is the recommended doses, although limited antitumor activity was observed (74). In light of these two recently concluded trials, combining sorafenib with conventional therapy may be a feasible option in the future, but more evidence is needed to ascertain the efficacy.
The addition of novel targeted therapy to conventional chemotherapy is a promising solution to overcome treatment resistance.
Combination of two targeted therapy agents
The combination of two targeted agents is another new direction due to the extensive crosstalk between the downstream signaling pathways (9-13) such that even if the primary pathway is blocked, activation of the complementary pathway can provide an avenue for resistance to the initial drug. Hence the ultimate goal of combination therapy is to inhibit two or more dominant targets responsible for tumor progression to overcome primary and secondary drug resistance. This is clearly shown in HER2-overexpressing breast cancer where anti-HER2 monoclonal antibody trastuzumab and the TK inhibitor lapatinib have demonstrated synergy in pre-clinical and clinical studies (75). In sarcoma, the combination of an mTOR inhibitor and IGF-1R inhibitors may hold promise. In a recently concluded phase I clinical trial investigating the preliminary efficacy of cixutumumab and temsirolimus, 20 patients with refractory ES were recruited and given cixutumumab (6 mg/kg IV weekly) and temsirolimus, (25 to 37.5 mg IV weekly), 4-week cycles for both. All tumors were restaged after 8 weeks. Results were encouraging. Toxicities were generally well tolerated, 7 (35%) achieved stable disease for more than 5 months or complete/partial responses, 5 (29%) patients had tumor regression of more than 20%, with 2 of the 5 achieving 100%. Of the 6 patients with ES who previously developed resistance to a different IGF-1R inhibitor antibody, 1 achieved complete response (76).
The same combination has also been tested in bone and soft tissue sarcomas. A recently concluded phase II study evaluated the safety and efficacy of cixutumumab and temsirolimus in these tumors, IGF-1R positive and negative. 383 patients were evaluated. Primary 12 weeks PFS endpoint was achieved (32% in IGF-1R positive STS, 38% in IGF-1R positive bone sarcoma, 43% in IGF-1R negative bone and soft tissue sarcomas). Western Blots of 32 matched pair tumors biopsies show inhibition of IGF-1R, pAKT and pS6. However, there was no correlation between plasma markers and PFS (77).
This is one example of a clinically realistic combination that identifies how combination therapy can overcome alternative pathway resistance. The potential of combination is clear however the details of the specific mechanism of resistance must be determined in preclinical studies.
Conclusions
Although sarcomas are initially or ultimately resistant to conventional chemotherapy in many cases, the emergence of targeted therapy agents as a new systemic treatment modality may offer hope to sarcoma patients. Single agents have shown activity and rational evidence based combinations may be able to overcome the resistances. This is an emerging area and is likely to produce additional improved patient outcomes over the next decade.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Thomas D, Whyte S, Choong P. Australian Sarcoma Study Group: development and outlook. Cancer Forum 2009;33:25-8.
- Ludwig J, Trent J. Targeted Therapy of Sarcoma. In: Kurzrock R, Markman M. eds. Targeted Cancer Therapy. Current Clinical Oncology™: Humana Press, 2008:317-34.
- Matushansky I, Taub RN. Adjuvant chemotherapy in 2011 for patients with soft-tissue sarcoma. Nat Rev Clin Oncol 2011;8:434-8. [PubMed]
- Krikelis D, Judson I. Role of chemotherapy in the management of soft tissue sarcomas. Expert Rev Anticancer Ther 2010;10:249-60. [PubMed]
- Gough NJ, Smith C, Ross JR, et al. Symptom burden, survival and palliative care in advanced soft tissue sarcoma. Sarcoma 2011;2011:325189.
- Yang JL, Crowe PJ. Targeted therapies in adult soft tissue sarcomas. J Surg Oncol 2007;95:183-4. [PubMed]
- Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 2005;315:971-9. [PubMed]
- Hopkins A, Crowe PJ, Yang JL. Effect of type 1 insulin-like growth factor receptor targeted therapy on chemotherapy in human cancer and the mechanisms involved. J Cancer Res Clin Oncol 2010;136:639-50. [PubMed]
- Chappell WH, Steelman LS, Long JM, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget 2011;2:135-64. [PubMed]
- Thomas D. Salvage strategies for relapsed or refractory acute lymphoblastic leukemia. Leukemia Insights 2010;15:1-8.
- Adjei AA, Hidalgo M. Intracellular signal transduction pathway proteins as targets for cancer therapy. J Clin Oncol 2005;23:5386-403. [PubMed]
- Meng J, Peng H, Dai B, et al. High level of AKT activity is associated with resistance to MEK inhibitor AZD6244 (ARRY-142886). Cancer Biol Ther 2009;8:2073-80. [PubMed]
- Chapman MS, Miner JN. Novel mitogen-activated protein kinase kinase inhibitors. Expert Opin Investig Drugs 2011;20:209-20. [PubMed]
- Baselga J, Norton L, Albanell J, et al. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998;58:2825-31. [PubMed]
- Malik F, Korkaya H, Clouthier SG, et al. Breast cancer heterogeneity: need to review current treatment strategies. Curr Breast Cancer Rep 2012;4:225-31.
- Rothschild SI, Gautschi O. Src tyrosine kinase inhibitors in the treatment of lung cancer: rationale and clinical data. Clinical Investigation 2012;2:387-96.
- Ljuslinder I, Melin B, Henriksson ML, et al. Increased epidermal growth factor receptor expression at the invasive margin is a negative prognostic factor in colorectal cancer. Int J Cancer 2011;128:2031-7. [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [PubMed]
- Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med 2012;63:247-58. [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [PubMed]
- Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [PubMed]
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [PubMed]
- Montemurro M, Gelderblom H, Bitz U, et al. Sorafenib as third- or fourth-line treatment of advanced gastrointestinal stromal tumour and pretreatment including both imatinib and sunitinib, and nilotinib: A retrospective analysis. Eur J Cancer 2013;49:1027-31. [PubMed]
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol 2010;28:1772-9. [PubMed]
- Stacchiotti S, Pedeutour F, Negri T, et al. Dermatofibrosarcoma protuberans-derived fibrosarcoma: clinical history, biological profile and sensitivity to imatinib. Int J Cancer 2011;129:1761-72. [PubMed]
- Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: Clinical development of a potent anti-angiogenic drug. Crit Rev Oncol Hematol 2011;77:163-71. [PubMed]
- Deeks ED. Pazopanib: in advanced soft tissue sarcoma. Drugs 2012;72:2129-40. [PubMed]
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879-86. [PubMed]
- Wang X, Crowe PJ, Goldstein D, et al. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers Int J Oncol 2012;41:1181-91. [PubMed]
- Wang X, Goldstein D, Crowe PJ, et al. S3I-201, a Novel STAT3 inhibitor, inhibits growth of human soft tissue sarcoma cell lines. World J Cancer Res 2013;1:61-8.
- Gentzler RD, Altman JK, Platanias LC. An overview of the mTOR pathway as a target in cancer therapy. Expert Opin Ther Targets 2012. [Epub ahead of print]. [PubMed]
- Blay JY. Updating progress in sarcoma therapy with mTOR inhibitors. Ann Oncol 2011;22:280-7. [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [PubMed]
- Vinayak S, Carlson RW. mTOR inhibitors in the treatment of breast cancer. Oncology 2013;27:38-44, 46, 48 passim. [PubMed]
- Thompson LA, Kim M, Wenger SD, et al. Everolimus: a new treatment option for advanced pancreatic neuroendocrine tumors. Ann Pharmacother 2012;46:1212-9. [PubMed]
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. [PubMed]
- Yao JC, Phan AT, Jehl V, et al. Everolimus in advanced pancreatic neuroendocrine tumors: the clinical experience. Cancer Res 2013;73:1449-53. [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256-65. [PubMed]
- Dickson MA, Schwartz GK, Antonescu CR, et al. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: clinical and molecular correlates. Int J Cancer 2013;132:1711-7. [PubMed]
- Subbiah V, Trent JC, Kurzrock R. Resistance to mammalian target of rapamycin inhibitor therapy in perivascular epithelioid cell tumors. J Clin Oncol 2010;28:e415. [PubMed]
- Chawla SP, Staddon AP, Baker LH, et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol 2012;30:78-84. [PubMed]
- Blay JY, Chawla SP, Ray-Coquard I, et al. Phase III, placebo-controlled trial (SUCCEED) evaluating ridaforolimus as maintenance therapy in advanced sarcoma patients following clinical benefit from prior standard cytotoxic chemotherapy: Long-term (≥24 months) overall survival results. J Clin Oncol 2012;30:abstr 10010.
- Zhang L, Hannay JA, Liu J, et al. Vascular endothelial growth factor overexpression by soft tissue sarcoma cells: implications for tumor growth, metastasis, and chemoresistance. Cancer Res 2006;66:8770-8. [PubMed]
- Rajendra R, Jones RL, Pollack SM. Targeted treatment for advanced soft tissue sarcoma: profile of pazopanib. Onco Targets Ther 2013;6:217-22. [PubMed]
- Rini BI. Targeted therapy for patients with renal-cell carcinoma. Lancet Oncol 2011;12:1085-7. [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [PubMed]
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol 2013;24:257-63. [PubMed]
- Rikhof B, de Jong S, Suurmeijer AJ, et al. The insulin-like growth factor system and sarcomas. J Pathol 2009;217:469-82. [PubMed]
- Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol 2010;11:129-35. [PubMed]
- Juergens H, Daw NC, Geoerger B, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol 2011;29:4534-40. [PubMed]
- Garofalo C, Manara MC, Nicoletti G, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing’s sarcoma is dependent on insulin receptor signaling. Oncogene 2011;30:2730-40. [PubMed]
- Pappo AS, Patel SR, Crowley J, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol 2011;29:4541-7. [PubMed]
- Malempati S, Weigel B, Ingle AM, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol 2012;30:256-62. [PubMed]
- Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12:89-103. [PubMed]
- Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res 2006;12:3657-60. [PubMed]
- Wagner AJ, Goldberg JM, Dubois SG, et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: results of a multicenter phase 2 trial. Cancer 2012;118:5894-902. [PubMed]
- Davis IJ, McFadden AW, Zhang Y, et al. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res 2010;70:639-45. [PubMed]
- Jun HJ, Lee J. Expression of MET in alveolar soft part sarcoma. Med Oncol 2010;27:459-65. [PubMed]
- Balke M, Hardes J. Denosumab: a breakthrough in treatment of giant-cell tumour of bone? Lancet Oncol 2010;11:218-9. [PubMed]
- Roux S, Mariette X. RANK and RANKL expression in giant-cell tumour of bone. Lancet Oncol 2010;11:514. [PubMed]
- Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 2010;11:275-80. [PubMed]
- Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 2005;65:671-80. [PubMed]
- Zahorowska B, Crowe PJ, Yang JL. Combined therapies for cancer: a review of EGFR-targeted monotherapy and combination treatment with other drugs. J Cancer Res Clin Oncol 2009;135:1137-48. [PubMed]
- Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 2005;315:971-9. [PubMed]
- Houghton PJ, Morton CL, Gorlick R, et al. Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program. Mol Cancer Ther 2010;9:101-12. [PubMed]
- Schuetze SM, Zhao L, Chugh R, et al. Results of a phase II study of sirolimus and cyclophosphamide in patients with advanced sarcoma. Eur J Cancer 2012;48:1347-53. [PubMed]
- D’Adamo DR, Anderson SE, Albritton K, et al. Phase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomas. J Clin Oncol 2005;23:7135-42. [PubMed]
- Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol 2012;23:785-90. [PubMed]
- Vincenzi B, Silletta M, Schiavon G, et al. Sorafenib and dacarbazine in soft tissue sarcoma: a single institution experience. Expert Opin Investig Drugs 2013;22:1-7. [PubMed]
- Martín-Liberal J, López-Pousa A, Broto JM, et al. Phase I trial of sorafenib in combination with ifosfamide in patients with advanced sarcoma: a Spanish group for research on sarcomas (GEIS) study. Invest New Drugs 2013. [Epub ahead of print]. [PubMed]
- Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633-40. [PubMed]
- Naing A, LoRusso P, Fu S, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin Cancer Res 2012;18:2625-31. [PubMed]
- Schwartz GK, Tap WD, Qin LX, et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: a multicentre, open-label, phase 2 trial. Lancet Oncol 2013;14:371-82. [PubMed]


