
Gallbladder cancer in South America: epidemiology and prevention
Introduction
Gallbladder cancer (GBC) is a biliary tract malignancy with a high incidence in specific regions of some developing countries (1), where its late diagnosis and ominous prognosis make GBC an important public health problem. Carcinogenesis is poorly understood due to the scarce knowledge of genetic and environmental factors. The present review provides an update of the epidemiology, the molecular genetics, the risk factors, the screening and the early diagnosis of GBC in South America (SA), focusing on high-risk areas. The goal is to improve our knowledge of this dismal disease, for which immediate public health decisions are required.
Epidemiology
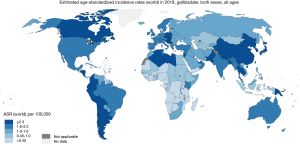
Based on current estimates GBC occupied the 20th place in incidence and the 17th place in mortality worldwide in 2018 [International Classification of Diseases 10° Revision (ICD10): C23-24] (Figure 1), representing 1.3% of new cancer cases and 1.7% of cancer-related deaths (excluding non-melanoma skin cancer) (1). Although C23-24 deaths are due to GBC and extrahepatic bile duct cancer, the majority correspond to GBC. GBC represents 4% of gastrointestinal cancers in both sexes; 6% in women and 3% in men. In Japan, based on the number of cases and not on rates, GBC accounts for 8% and 5% of gastrointestinal cancers in women and men, respectively. Indeed, incidence and mortality age-standardized rates (ASR) are higher in females worldwide (2.4 and 1.8 per 100,000 females per year, and 2.2 and 1.6 per 100,000 men per year, respectively), except in some Asian countries like Republic of Korea and Japan where the highest ASR values were in men (1).
Due to demographic changes, the burden of GBC is expected to increase all over the world by more than 75% (165,600 new cases and 130,400 new deaths) by the year 2040.
Incidence and mortality of GBC show significant variation worldwide (1,2). The highest estimated ASR is over 2 per 100,000 per year in countries from Eastern Asia (over 2.7) and the west part of SA. While, the regions of North America, Northern Europe, Australia, and New Zealand show incidence and mortality ASRs around 1 and even lower (Figures 1,2).
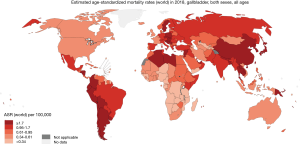
In SA, 15,114 new GBC cases and 11,097 GBC-related deaths were estimated for the year 2018. Incidence and mortality ASR exhibit striking geographic variation, reaching epidemic levels particularly in the Andean Area (3,4). The highest incidence and mortality ASRs have been reported in Bolivia (14.0 and 10.6, respectively), Chile (9.3 and 5.4) where GBC is the second leading cause of death from cancer in women, and Peru (4.8 and 3.1). In these countries, GBC mortality showed an unequal distribution. The areas with the highest values were: the south-inland region in Chile; the region near Lake Titicaca in Bolivia and the area of Trujillo city in Peru (5). GBC is more frequent among female indigenous people (Mapuche) and in people living in areas with high poverty and insufficient access to health services (Figure 3) (6-10).

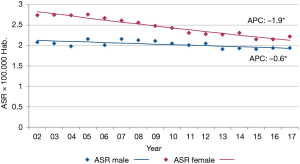
The International Agency for Research on Cancer (IARC) estimates GBC incidence in Argentina from population-based cancer registries (PBCR) that have reached the standards for data quality. In 2018, the incidence ASR was 2.7 in males and 2.5 in females for the whole country (1). However, these rates are based on five registries, those with high-quality data, and they do not take into account the heterogeneous distribution of GBC inside the country. The geographic variation is shown with the analysis of GBC mortality, instead of incidence because it is much more comprehensive encompassing the twenty-three provinces and the federal district. The latest official information comes from the 2017 DEIS (Department of Statistics and Health Information, for its acronym in Spanish) mortality database. In this year a total of 1.285 deaths were registered as GBC (ICD 10: C23-24) all over the country, with a mean age of 69 (95% confidence interval: 68–70) in both sexes. The ASRs were 1.9 in men and 2.2 in women, with no statistically significant difference. However, there was a high geographic variation. In both sexes the highest mortality ASRs (quintile with the highest value range), were observed in the northwestern provinces of Jujuy (6.6 in females and 6.4 in males) and Salta (7.6 in females and 4.2 in males); in both regions GBC is the third leading cause of death from cancer in women. Age-standardized rates were followed in men by the western provinces of San Luis and Tucuman (3.3 in both), and in women by the western province of Neuquén (6.9) and the southern province of Tierra del Fuego (5.9) (Figure 4). In contrast, the provinces at the east of the country showed mortality ASR from 1.2 to 1.9 per 100,000 women per year and from 0.6 to 1.7 per 100,000 men per year (quintile with the lowest value range). GBC mortality trends decreased over the period 2002–2017 in both sexes. The estimated annual percentage of change (APC) at any age was −1.9% in women and −0.6% in men, both reaching statistical significance (Figure 5). In the provinces with the highest mortality ASR (Jujuy, Salta, and Neuquen) the decrease was observed in both sexes, with the exception of Salta where the mortality ASR increased in men of any age, and in men and women over 50 years old (Atlas 2002–2016 not published). Actually, a consistent increase in the age-specific mortality rate has been observed in men and women older than 50 years all over the country (Atlas trends 2002–2016, not published).

The distribution of GBC mortality ASR by departments in Argentina (provinces are politically divided in departments), shows a pattern similar to provinces, with values two hundred percent higher than the national average in poor areas at the north of the country as well as in the Andean regions (11) (Figure 6).

GBC is a highly lethal disease. Approximately 30% of patients with lesions confined to the gallbladder wall survive 5 years and only 10% with more advanced stages survive 1-year (12,13). The majority of cases are diagnosed at later stages, and this explains the high lethality.
The mortality/incidence (MI) ratio is another way to estimate the survival of cancer patients. Interestingly, there is a great disparity in the GBC MI ratio worldwide. Based on this ratio the survival is around forty and twenty percent in high-income and middle-income countries, respectively. The intermediate range is observed in low-income countries (Table 1). This disparity could be due to differences in socioeconomic status, education level, and life expectancy. Also, the MI ratio varies according to sex. With the exception of Chile, survival rates worldwide (based on GBC MI ratios) were higher in men, which suggests that GBC in women is not only more prevalent but also more lethal.

Full table
Risk factors
Several risk factors for GBC have been identified, many of which share a common feature of chronic inflammation of the gallbladder (14-16). Other risk factors are age, female gender, genetic characteristics, socioeconomic status, and dietary patterns. The main risk factors are summarized in Table 2.

Full table
Gallstone disease
Gallstone disease or gallbladder lithiasis is present in 70 to 90 percent of patients with GBC (16-18). A history of gallstones appears to be one of the strongest risk factors for the development of GBC (18-20). A case-control study from Shanghai showed a 34-fold increase in the risk of GBC in patients with gallbladder lithiasis (16). One autopsy data-based study from Chile suggested that the risk of GBC in patients with gallstones is seven times greater compared to patients without it.
The overall incidence of GBC in patients with gallstone disease is only 0.5 to 4 percent. The risk increases with the duration of lithiasis and the size of gallstones (21,22). Indeed, the relative risk of GBC in patients with gallstones is 2.4 if the diameter is 2.0–2.9 cm, but increases to 10.1 if larger than 3.0 cm (16).
Since 10% to 30% of GBC cases do not have gallstone disease and only a small proportion (0.5–4%) of patients with gallbladder lithiasis develops GBC (23-26), other factors may be important in the development of GBC. For example, some ethnic and racial groups show an incidence of GBC higher than expected for their frequency of gallstone disease, suggesting that other environmental or genetic factors may also play a role in the course of carcinogenesis (27).
Porcelain gallbladder
Porcelain gallbladder is detected in 0.06 to 0.08 percent of cholecystectomy specimens (28). It has a female predominance and the incidence of GBC in patients with porcelain gallbladder is between 2 and 3 percent (29).
Patients with selective mucosal calcification of the gallbladder wall appear to be at higher risk for GBC compared with those in whom the gallbladder wall is completely calcified (complete type) (28-31).
Primary sclerosing cholangitis (PSC)
PSC is a chronic fibro-inflammatory syndrome which has a close association with the chronic inflammation and the carcinogenesis. There is an increased frequency of GBC in patients with PSC, which in all likelihood correlates with the ongoing inflammation, facilitating a metaplasia-dysplasia-carcinoma sequence (32,33).
Anomalous pancreaticobiliary duct junction
Several studies have suggested that an anomalous pancreaticobiliary duct junction (APBDJ) is associated with the development of GBC (34-36). Patients with GBC and APBDJ are generally young, have a low incidence of gallstones, and their tumors show a higher frequency of K-ras mutations (37,38). The anomalous ductal union is seen in about 17% of patients with GBC compared with less than 3% in patients with other hepatobiliary disorders. In the APBDJ the pancreatic duct ends into the common bile duct outside the duodenal wall and not under the control of the sphincter; therefore, pancreatic juice can freely flow back into the gallbladder causing bile stasis, which leads to precancerous changes in the gallbladder mucosa. In APBDJ cases without dilatation of the bile duct, the risk for GBC is higher compared with those with bile-duct dilatation.
Chronic infection
In endemic areas, 1–4% of acutely infected individuals become chronic asymptomatic carriers of Salmonella Typhi (S. Typhi). One meta-analysis of 17 case-control and cohort studies suggested an association between chronic S. Typhi carriage and elevated risk of GBC (39). A study performed in Mexico and Bolivia concluded that patients diagnosed for typhoid disease had a twelve fold higher risk of developing GBC (40).
Geography/ethnicity
GBC has a wide geographic variation, as previously described (1,41). GBC particularly afflicts some indigenous populations (27). We will describe this topic in more detail later, in “Genetics” item.
Socioeconomic status
In a case-control study of 228 people from Chile by Serra et al., very low socioeconomic status was associated with GBC, with an odds ratio (OR) of 6.3 (95% CI: 1.7–23.0) (22). It is conceivable that people with very low socioeconomic status have less access to the health care system and, therefore they are more likely to have longstanding gallstone disease.
Sex
As described above, GBC predominates in women over men worldwide. Females having early menarche, late menopause, multiple pregnancies, and childbirths appear to have an increased risk for developing GBC according to a case-control study from India (42). Interestingly, estrogens increase the formation of gallstones, mainly by elevating biliary cholesterol (43).
Obesity
Overweight has been consistently associated with an increased risk for GBC. For each 5-point increment in body mass index, the relative risk increases by 1.59 in women and 1.09 in men (44).
Genetics
GBC ensues from an interaction between an innate genetic predisposition and exposure to environmental risk factors. Genetic factors undoubtedly govern some of the widely variable frequencies of GBC worldwide.
The contribution of the genetic background in the development of GBC emerges from the hypothesis about the migratory phenomenon from high incidence areas in Chile and Bolivia to Argentina. The northwestern region in Argentina shares borders with the Chilean region of Antofagasta and GBC mortality rates are similar on both sides of the Andes Mountains. Similarly, in the province of Neuquen in the western south of Argentina, mortality rates due to GBC are among the highest in the country. In this area the ancestral Pewenches were invaded by the Mapuches, coming from a southern region of Chile with the highest mortality rates of GBC, establishing a bidirectional population flow that still happens nowadays. Consequently, it is tempting to speculate that the high mortality rates of GBC in Neuquen are partially due to the migration of Mapuches from Chile (45). Also, the northern provinces of Salta and Jujuy where GBC is the third leading cause of death from cancer in women, have been receiving migrating people from Bolivia for decades, where GBC mortality is very high.
Lorenzo Bermejo and others investigated the relationship between the type of Native American ancestry and the main causes of death. Based on the genotypic genome data of 1805 Chileans, they showed an association between Mapuche ancestry and GBC; every 1 percent increase in Mapuche ancestry represented a 3.7 percent increase in the risk of death from GBC (95% CI: 3.1% to 4.3%, P=6×10−27) (45). For the first time, a strong association between ancestry and risk of GBC is shown and supports the migratory phenomenon described above as the main reason for the increased risk of GBC in the western province of Neuquen in Argentina.
Aflatoxin
Several investigations have shown an elevated concentration of aflatoxin in red chili peppers which are consumed in Andean areas from Chile, Bolivia Peru with the highest GBC incidence ASR (46,47). Nogueira et al. (48) performed a case-control study with 112 Chilean men and women, and found that patients with GBC had higher levels of aflatoxin B1-DNA adducts in peripheral blood compared with healthy control individuals (OR 13.2; 95% CI: 4.3–47.9) and with control individuals with gallstones (OR 9.4; 95% CI: 2.8–37.2). Koshiol et al. also studied this association in a case-control study from Shanghai, China (49). The plasma level of aflatoxin B1 (AFB1-lysine adduct) was found three more times in patients with GBC compared with controls (OR, 2.71; 95% CI: 1.70–4.33). The population attributable fraction for cancer-related to aflatoxin was 20% (95% CI: 15–25%).
In summary, there is evidence to consider red chili pepper, a frequently consumed food in some regions of SA, as a risk factor for GBC. This, in turn, could be mediated by the aflatoxin mutagen, a contaminant of red chili pepper.
Age
GBC rates tend to increase with advancing age (50). In the 2017 DEIS mortality database from the entire country of Argentina, age-specific GBC mortality rates increased consistently with age, the peak age of death was 80 and over (289/1,285), and 7 percent of deaths (88/1,285) were in patients younger than 50 years old. An analysis of sixty cases from provinces at high risk in Argentina (ILOGI database, data not published) also revealed an increment of GBC mortality with increasing age. However, the peak age of death was 54–59, and 17 percent of deaths occurred in people under 50 years old.
Tobacco and alcohol
Smoking and alcohol (40) have been described as risk factors for GBC, although with a low level of evidence. Recently McGee reported a meta-analysis from 26 prospective studies to evaluate associations of cigarette smoking and alcohol consumption with biliary tract cancer (51). Although current smoking was associated with intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma (ECC) and ampulla of Vater cancer (AVC), no convincing association was observed between smoking and GBC. Alcohol consumption was only associated with ICC. Therefore, the evidence is not sufficient to state that alcohol or tobacco consumption is a risk factor for GBC.
Molecular biology
The molecular biology of biliary cancer, including GBC, is poorly understood (52). The information available is very limited (53) even more in SA, is based in retrospective data with a low number of samples, and is not representative of majorly affected racial-ethnic groups.
While there is consensus regarding chronic epithelial inflammation as an initial pathogenic event (54), the mechanism of tumor progression is not clearly defined (55). Two models have been postulated: metaplasia to dysplasia, and adenoma to dysplasia. The first shows alterations in tumor suppressor genes (CDKN2A, p53, p57, KIP2), adhesion molecules (CEA, CD99), oncogenes (C-erb-B2, KRAS) and others genes (hTert, VEGF, Muc-1, iNOS, COX2, MGMT) (56,57). In the adenoma-carcinoma model, alterations in cell adhesion molecules (CD54 and CD56) and mutations of the CTNNB1 gene have been reported (58,59). The model of metaplasia-dysplasia has greater acceptance because these histologic alterations are more frequent in GBC (60% and 90% respectively) (60) compared to adenomatous polyps in less than 3% of specimens obtained from early GBC (61).
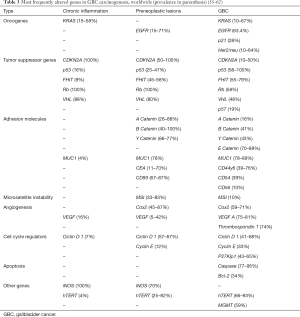
As in other tumors, GBC carcinogenesis involves multiple genetic alterations. Tumor suppressor genes, oncogenes, DNA repair gene mutations, microsatellite instability (MSI) and epigenetic alterations are reported with greater frequency and wide variation (62). Genetic abnormalities in GBC worldwide are depicted in Table 3. We will describe the main molecular abnormalities in GBC reported from SA (Table 4).
Full table

Full table
Genetic alterations
Tumor suppressor genes
In 42 cases of GBC from Chile (20/42, 48%) and Japan (22/42, 52%), mutations in exons 5 to 8 which deregulate the p53 gene were reported (63). In the Chilean patients, they found at least 1 mutation in 55.5% (11/20) of the cases and described 13 different mutations. There was geographical variation: C:G to A:T transition and C:G pair mutation mainly in Chilean patients, and A:T pair mutation predominantly in patients from Japan.
Wistuba et al. studied the p53 protein overexpression by immunohistochemistry in normal epithelium and premalignant lesions in patients from Temuco, a southern area in Chile with a very high incidence of GBC (64). Normal and metaplastic epithelium did not overexpressed p53. On the contrary, the protein was overexpressed in 34 of 52 (65.4%) GBC, 21 of 47 (44.7%) carcinomas in situ, and 11 of 34 (32.4%) dysplastic tissues. This suggests that p53 inactivation is an early event in the pathogenesis of GBC.
Asay et al. (65) reported overexpression of p53 in 18/36 (50%) GBC patients from Bolivia, a frequency similar to other world areas with high GBC incidence (66).
Roa et al. found inactivation of the CDKN2A gene in 13/38 (34%) cases of GBC from Chile. In 9/38 (24%) samples it was produced by methylation in promoter areas, while in 4/38 (11%) by allelic imbalance. Although not statistically significant, the inactivation of p16 (the main product of CDKN2) was more frequent in the Mapuche ethnic group and males. It was associated with ominous prognosis (P=0.09) (67).
ILOGI (68) (for its acronym in Spanish, Latin-American Intergroup of Gastrointestinal Oncology) analyzed the mRNA levels of the FBXW7 gene (also known as hCdc4 tumor suppressor gene) in 64 BC samples, the majority with GBC (27 GBC, 26 CC, 6 VBT, and 5 BC not specified) from Argentina (69). A correlation with survival was reported. Median progression-free survival was 4.9, 7.6 and 26.9 months for low, medium and high FBXW7 expression tertiles, respectively. OS was 6.2 months in the lowest tercile, 8 in the intermediate tercile, and not reached in the highest tercile. No FBXW7 hotspot mutations were detected.
Oncogenes
Activation of the RAS family is one of the most commonly reported events in GBC (66-70). A study of 25 GBC cases from Chile (71) described genetic alterations related to tumor progression RAS gene point mutations were reported in 2 cases (2/21, 10%), 1 K-ras and 1 N-ras in codon 12 and 61 respectively. All of them were found in invasive and poorly-differentiated tumors.
Discordant results of K-ras mutations were reported in neighboring regions of SA. These abnormalities were found in GBC in 29–30% (10/33, 10/35) of cases in Chile (72,73), 17% (10/58) in Argentina (54), 2.8% (1/36) in Bolivia (65) and 0% (0/30) in Peru (74). For comparison outside SA, in India, Korea, and Europe K-ras mutations were described in 41% (16/39), 20% (3/15) and 5% (1/20) of cases, respectively (75,76). It would be interesting to study the prevalence of biliopancreatic duct malformations in these populations since published series suggest a relationship of K-ras mutations to this congenital abnormality, stronger than the association of K-ras mutations with biliary lithiasis (77).
In Chile, Roa et al. reported the largest study of Her2/neu in GBC in SA (78). Utilizing the most accepted IHC criteria for breast cancer they found expression of Her2/neu in 24/187 (12.8%) cases. Patients with HER2/neu overexpression showed a lower 5-year overall survival, in comparison to those without overexpression (34% versus 41%, not statistically significant). Overexpression was more frequent in advanced cancers (13.8%) and well-differentiated tumors (17.4%), although this difference was not statistically significant.
Arroyo et al. (from ILOGI) reported over-expression of the c-MET protein in 25/52 (48%) GBC samples from Argentina (79). Patients with this molecular abnormality had a median overall survival of 11.3 months compared to 18.2 months in patients without it (P=0.068). Over-expression correlated with tumor grade (P=0.015), showing an inverse association. In EEUU, the MET amplification was reported in low frequency (1%) in a series of 85 GBC cases studied with a next-generation sequencing platform (80).
Other molecular-genetic alterations
Arroyo et al. analyzed the expression of the KIT protein in GBC cases from northwestern Argentina (81). Immunohistochemistry revealed a 3+ score in only 3/50 samples (6%), all being poorly differentiated tumors. Went et al. reported similar results, with no expression in 27 GBC samples from European patients (82). In contrast, weak and moderate KIT expression was described in 7/12 (58%) biliary tract cases from the EEUU (83), with no discrimination between GBC and cholangiocarcinoma.
ILOGI studied the expression of multidrug resistance-associated protein 4 (MRP4) in 15 GBC samples from Salta and Jujuy, the provinces with the highest mortality rates of GBC in Argentina (Figure 4) (11). MRP4 overexpression increases cell proliferation, tumor growth and chemoresistance in pancreatic cancer (84). Ten out of fifteen (66%) samples showed high expression, and this was associated, although not statistically significant, with shorter overall survival (85). These findings led to an expansion study which is in progress.
Genomic imbalances
Roa et al. studied the content of DNA in 112 samples of GBC from Chile, by flow cytometry (86). They found aneuploidy in 26% of cases (29/112) and this abnormality was associated with lymphatic tumor involvement: 73%, 16/22, in aneuploid tumors versus 48%, 22/46, in diploid tumors (P=0.05).
Epigenetic alterations
Although less frequently studied, epigenetics has a relevant role in gallbladder carcinogenesis (87). DNA methylation is the most commonly reported event of epigenetics in GBC (59). García et al. in Chile published a series of 102 samples with distinct evolutionary stages (cholecystitis, metaplasia, GBC) (88). They found a significant relationship between survival and methylation of MGMT and DCL1 genes, with better survival for the first case (P=0.006) and poor prognosis for the second (P=0.04). They also showed a progressive methylation index from cholecystitis to advanced GBC in DAPK1, DLC1, TIMP3, and RAR beta2 genes (P≤0.05) indicating that aberrant hypermethylation of promoter regions is an early, progressive and cumulative event in gallbladder carcinogenesis. The methylation status of some individual genes could be utilized as a biomarker with potential clinical applications in diagnosis or prognosis of GBC if they are validated in a greater number of samples.
MSI
High MSI (MSI-H) has been reported in 6/59 (10%) GBC samples from Temuco, Chile, with equal distribution between early and late stages (89). It was also found in 2/6 (33%) samples with metaplasia and 5/6 (83%) with dysplasia. Similarly, one study from Japan found MSI in 9/30 (30%) samples with cholecystitis, 7/17 (41%) with carcinoma, 5/15 (33%) adjacent non-neoplastic mucosa (90). It seems that MSI is an early event in GBC carcinogenesis. This requires confirmation in larger series.
Screening and prophylactic cholecystectomy (PC)
Therapy for metastatic GBC is minimally effective. Prevention (primary and early detection) is the ideal strategy to decrease the rising death rates from this fatal tumor. Prevention requires a thorough evaluation of risk factors and identification of high-risk asymptomatic subgroups of patients in whom PC could be beneficial.
The serum markers CD34, Ca15-3, MIB-1, CEA, and Ca19-9 have been tested for the early diagnosis of GBC (91-93). Although some of them can contribute to the diagnosis of suspicious cases and the prognosis of GBC, the very low specificity precludes their utilization for early diagnosis. Even more, none of these serum markers has been validated for this purpose in a clinical setting.
Cholecystectomy avoids the development of GBC (primary prevention) and treats GBC at the early stages when it is found incidentally (secondary prevention). Indeed, five-year survival rates for mucosal (T1a) and submucosal (T1b) invasive GBC are 94% and 89%, respectively (94,95). Also, two series from SA described five-year survival rates of 50% and 53% for incidental GBC overall (96,97). Therefore, cholecystectomy in patients at risk is a potential strategy for prevention.
Several studies have shown a correlation between rates of cholecystectomy (the majority for treating symptomatic gallstone disease) and GBC incidence or mortality (98-100). Chianale et al. (99) studied a possible correlation between the GBC mortality and the cholecystectomy rates during a decade in Chile. The results suggested that changes in the number of cholecystectomies performed in a specific geographic area would reciprocally change the death rate from GBC. They estimated that an increase in the number of cholecystectomies by 12,500 per year would decrease the death rate from this cancer by approximately 1.0 per 100,000 people in Chile.
Alexander et al. (100) studied all patients diagnosed with GBC in the area of the Dutch Eindhoven Cancer Registry between 1975 and 2008 (n=659). They observed that the incidence ASR of GBC in the south of the Netherlands has declined drastically along these three decades, and concluded that this is probably associated with a rise in the number of cholecystectomies during those years.
Diehl et al. (101) analyzed GBC mortality and its relationship with cholecystectomy rates. The data was taken from the national health statistics of England and Wales, Scotland, EEUU, Sweden, Canada, and Australia during 1967–1977. Trends in GBC mortality were inversely associated with trends in cholecystectomy rates, the only exception being women in Canada.
All these studies have been performed in low-risk populations and do not have cost-effectiveness analyses. They did not compare GBC mortality in patients with asymptomatic gallbladder stone disease who underwent cholecystectomy versus those who did not. Also, although gallstone disease is the main risk factor for GBC many others can determine variations in the incidence and the mortality. Therefore, the evidence that these studies generate is weak to establish cholecystectomy as a standard procedure for the prevention of GBC. Even more, studies based on decision analysis models concluded that the role of cholecystectomy in asymptomatic patients for the prevention of GBC (PC) was not better than the current recommendation for expectant management of gallstone disease (102-105).
A way to improve the results of PC for the prevention of GBC is to select a population with other risk factors that in addition to gallstone disease or because of their intrinsic role, confer a higher risk of GBC. Some of these factors are polyps larger than 10 millimeters (106), porcelain gallbladder (29), stones larger than three centimeters (21), ethnic groups like Mapuches (45), and people living in areas at high risk for GBC (33-45). However, the evidence to support the use of PC in these subgroups of patients is not sufficient.
In Chile, where GBC is the second leading cause of death from cancer in women, there is a national program, named explicit health guarantees (EHG) which utilizes PC intending to decrease mortality from GBC (107). The rationale is to remove the target organ at an age when the prevalence of lithiasis is high and considering that the latency period between acquisition of gallstones and cancer is estimated in 7 to 8 years (108). Screening with ultrasonography is performed in the following groups in the general population (Figure 7):
- Men and women, age 35 to 49 years, with symptomatic lithiasis.
- Men and women, age 35 to 49 years, asymptomatic, with any of the following risk factors: multiparity, body mass index equal to or greater than 27, less than 8 years of formal education, or Mapuche ethnicity that includes at least one surname corresponding to this genealogical origin.
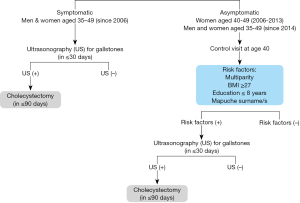
If the ultrasonography reveals gallstone disease the patient undergoes PC. If there is no lithiasis, the patient is not operated.
Since the implementation of the EHG program in 2006, the number of cholecystectomies has progressively increased (55,665 hospital discharges due to cholecystectomy in 2006; 75,882 in 2012). However, the age-adjusted mortality rate of GBC, including the age group targeted by the intervention, decreased before the plan started, from 12.57 per 100,000 inhabitants in the year 2000 to 8.17 in 2012 (109). This trend may be due to other factors like improvements in environmental and socio-economic sanitation. Problems observed with the EHG program are the following: it has not yet achieved an acceptable level of cholecystectomies, has not included women over 50 years old (a subgroup with very high risk), and has not prioritized patients from regions with the highest prevalence of GBC, which are in the South of Chile (109,110).
In spite of this, some cost-effectiveness studies and statistical modeling suggest that the strategy of programs like EHG would be effective (decrease in GBC mortality) at an acceptable incremental cost (111). PC has the additional benefit of preventing the inflammatory and infectious complications of gallstone disease (acute and chronic cholecystitis, acute pancreatitis, obstructive jaundice) with the consequent reduction in the associated morbidity and mortality and the socio-economic impact they have (112). However, other cost-effectiveness analyses reached different conclusions. Kapoor et al. (111) performed this type of study in the state of Uttar Pradesh in India, which has a population of two hundred million and a prevalence of gallstone disease of four percent (eight million patients). If one center has to perform five PC daily over two hundred working days per year, eight hundred such centers working for ten years would be required to treat every patient in the country. With a 0.5% risk of bile duct injury, this could result in forty thousand bile duct injuries.
Discussion
The patterns of distribution of risk factors to GBC are different worldwide and by regions. This is the case for Argentina wherein GBC mortality ASR for the entire country in women and men was 2.2 and 1.9 respectively in 2017 constituting the tenth and the fourteen leading cause of death from cancer in women and men, respectively. But in the northwestern provinces of Jujuy and Salta, it was much higher (6.6 and 7.6 in women and men, respectively) with GBC reaching the third place in women and the fourth in men in the leading causes of death from cancer. Argentina is a very extensive country with important differences in ethnicity, environment, cultures and socioeconomic status among its distinct regions. This, in turn, produces an important geographic variation in the prevalence of risk factors. For instance, Mapuche ancestry, a risk factor for GBC, is much more prevalent in the province of Neuquen where the GBC mortality ASR in women is 6.9. Although not proven, it is possible that migration from Bolivia (the country with the highest GBC mortality in SA) to the northwestern provinces of Salta and Jujuy in Argentina has been introducing a genetic risk factor. Also important, Salta and Jujuy are among the poorest provinces in Argentina. Indeed low socioeconomic status is a risk factor for GBC probably because the low access to health services contributes to longstanding gallstone disease, a well-known risk factor for GBC. Another risk factor that is distributed not homogeneously is the consumption of red chili pepper, which is more prevalent in regions from the western countries of Chile, Bolivia, and Peru, where mortality due to GBC is the highest in SA. The wide geographic variation in the incidence and mortality of GBC in SA requires that epidemiological analyses be carried out by regions and not by countries. This would allow the identification of areas at high risk for GBC where public health programs and resources must be applied. Examples of these regions are the western provinces of Argentina, some southern regions of Chile and the area near Trujillo city in Peru.
As cancer is a multifactorial disease further research is required to find new risk factors that explain the particular distribution of GBC in SA, and contribute to the implementation of preventive measures. Also as GBC has particular risk factors that are not shared by other biliary cancers (ICC, ECC, and AVC), the anatomic origin of biliary tract cancer has to be taken into account in registries as well as in molecular and clinical studies, where GBC should be analyzed in a separate way
In Argentina, GBC mortality ASR has been decreasing from 2002 to 2017, more in women. This could be related to the increment in the number of cholecystectomies due to gallstone disease along those years since 1 to 3 percent of these operations find an incidental GBC (23-25). However, a decrease in other risk factors may have contributed to the observed decrement in GBC mortality as well.
Aging is a risk factor for GBC. Also, analysis of the data mentioned above, suggests that people with GBC in high-risk regions are younger in comparison to patients with GBC in other areas. If this is confirmed in larger series it would suggest a genetic predisposition in areas at high risk, since it is known from other tumor models that cancer cases with genetic predisposition affect people at a younger age (112).
Gallstone disease is the main risk factor for GBC (70 to 90 percent of the cases in high-incidence regions from SA). Fortunately, only 0.5 to 4 percent of the patients with lithiasis develop GBC and other risk factors also contribute to the susceptibility to this disease. The carcinogenesis of GBC is a multistep, multifactorial process (113), which has to be explored in-depth in order to identify novel risk factors that permit to stratify individuals according to their GBC risk and improve the efficiency of prevention strategies. The current prevention programs rely on the PC offered to patients with gallbladder lithiasis. However, even if additional risk factors like sex, obesity, Native American ancestry, multiparity, and educational level are considered, the cost-effectiveness of PC for the general population is questionable. To optimize the prevention of GBC in regions of high and low incidence, a team of European-Latin American researchers and clinicians from Argentina, Bolivia, Chile, Peru, France, Norway and the UK led by Prof. Dr. Lorenzo Bermejo in Germany aim to conduct an ambitious research project. The final objective is to investigate the potential of circulating biomarkers (small non-coding RNAs among others) through the model of GBC carcinogenesis represented by the sequence of normal gallbladders with stones and inflammation, low-grade dysplasia, high-grade dysplasia, in situ carcinoma and invasive carcinoma. The underlying hypothesis is that each step of GBC development is reflected in unique patterns of circulating biomarkers, leading to more efficient strategies for GBC prevention. Patients with a high individual risk of developing GBC should be prioritized for undergoing PC.
The heterogeneity of molecular biology reports of GBC probably is due to the different evolutionary steps of carcinogenesis at which studies were performed, the low number of cases, the different methods for analysis and the mixing of biliary tumors from the different anatomic locations. Also, GBC, ICC, ECC, and AVC have different molecular abnormalities (57). In order to comprehensively study the molecular alterations of GBC in SA, ILOGI and Phoenix Mayo Clinic are analyzing 58 samples from high-risk areas in Argentina in more than 150 genes. The findings will be correlated with clinical characteristics and survival. Since the prevalence of risk factors is distinctive in regions of SA with high-risk for GBC it is possible that molecular abnormalities are different in these areas in comparison to other regions of the world.
In conclusion, GBC is a significant problem for public health in western regions of SA. The treatment of GBC is not effective and GBC prevention constitutes the most efficient strategy to decrease mortality. PC is a promising preventive measure but its cost-effectiveness has to be optimized. The future identification of novel biomarkers of GBC risk combined with established risk factors will permit effective implementation of GBC prevention programs to decrease the high mortality of GBC observed in western SA.
Acknowledgments
Professor Justo Lorenzo for his collaboration.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer 2018 Available online: https://gco.iarc.fr/today, consulted May 2019.
- Hundal R, Shaffer E. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99-109. [PubMed]
- Izarzugaza MI, Fernandez L, Forman D, et al. Burden of gallbladder cancer in Central and South America. Cancer Epidemiology 2016;4 sup 1:S82-9.
- Muñoz P, Vidal C, Moya P, et al. Mortality trend of gallbladder cancer in Araucanía Region, Chile, 2004-2014. Medwave 2017;17:e7035. [Crossref] [PubMed]
- Ikoma T, Tsuchiya Y, Asai T, et al. Ochratoxin A Contamination of Red Chili Peppers from Chile, Bolivia and Peru, Countries with a High Incidence of Gallbladder Cancer. Asian Pacific Journal of Cancer Prevention 2015;16:5987-91. [Crossref] [PubMed]
- Moore SP, Forman D, Piñeros M, et al. Cancer in indigenous people in Latin America and the Caribbean: a review. Cancer Medicine 2014;3:70-80. [Crossref] [PubMed]
- Bertran E, Heisi K, Andia ME, et al. Gallbladder cancer: incidence and survival in a high-risk area of Chile. Int J Cancer 2010;127:2446-54. [Crossref] [PubMed]
- Tairo-Cerron T, Paredes-Orue R, Moreno-Loaiza O. Frequency and characteristics of gallbladder cancer at a referral hospital in southern Peru, 2009-2014: a descriptive study. Medwave 2018;18:e7184. [Crossref] [PubMed]
- Uribe MM, Heine CT, Brito FM, et al. Gallbladder cancer: an update. Rev. Med. Clin. Condes 2013;24:638-43.
- Navarro Rosenblatt D, Durán Agüero S. Gallbladder cancer and nutritional risk factors in Chile. Nutr Hosp 2016;33:105-10. [PubMed]
- Macías G, Limardo L, Abriata MG. Atlas of Cancer Mortality: Argentina, 2011-2015, 1st ed. Autonomous City of Buenos Aires: National Cancer Institute 2017.
- Mahdavifar N, Pakzad R, Ghoncheh M, et al. Epidemiology, incidence, and mortality of gallbladder cancer and its relation with development in the world. Ann Trop Med Public Health 2017;10:563-70.
- Lazcano-Ponce EC, Miquel J, Muñoz N, et al. Epidemiology and Molecular Pathology of Gallbladder Cancer. CA Cancer J Clin 2001;51:349-64. [Crossref] [PubMed]
- Caygill CP, Hill MJ, Braddick M, et al. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 1994;343:83-4. [Crossref] [PubMed]
- Diehl AK. Epidemiology of gallbladder cancer: a synthesis of recent data. J Natl Cancer Inst 1980;65:1209. [PubMed]
- Hsing AW, Gao YT, Han TQ, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer 2007;97:1577. [Crossref] [PubMed]
- Maringhini A, Moreau JA, Melton LJ 3rd, et al. Gallstones, gallbladder cancer, and other gastrointestinal malignancies. An epidemiologic study in Rochester, Minnesota. Ann Intern Med 1987;107:30. [Crossref] [PubMed]
- Paraskevopoulos JA, Dennison AR, Ross B, et al. Primary carcinoma of the gallbladder: a 10-year experience. Ann R Coll Surg Engl 1992;74:222. [PubMed]
- Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst 1997;89:1132. [Crossref] [PubMed]
- Chow WH, Johansen C, Gridley G, et al. Gallstones, cholecystectomy and risk of cancers of the liver, biliary tract and pancreas. Br J Cancer 1999;79:640. [Crossref] [PubMed]
- Diehl AK. Gallstone Size and the Risk of Gallbladder Cancer. JAMA 1983;250:2323-6. [Crossref] [PubMed]
- Serra I, Yamamoto M, Calvo C, et al. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a chilean population. Int J Cancer 2002;102:407-11. [Crossref] [PubMed]
- Koppatz H, Nordin A, Scheinin T, et al. The risk of incidental gallbladder cancer is negligible in macroscopically normal cholecystectomy specimens. HPB 2018;20:456-61. [Crossref] [PubMed]
- Muszynska C, Lundgren L, Lindell G, et al. Predictors of incidental gallbladder cancer in patients undergoing cholecystectomy for benign gallbladder disease: Results from a population-based gallstone surgery registry. Surgery 2017;162:256. [Crossref] [PubMed]
- Ghimire P1. Yogi N, Shrestha BB. Kathmandu Incidence of incidental carcinoma gall bladder in cases of routine cholecystectomy. Univ Med J 2011;9:3-6. (KUMJ).
- Andia M, Gederlini A, Ferreccio C. Cáncer de vesícula biliar: Tendencia y distribución del riesgo en Chile. Rev Med Chi 2006;134:565-74. [Crossref]
- Surveillance, Epidemiology and End-Results Program (SEER). The Four Most Common Cancers for Different Ethnic Populations 2013. Bethesda, MD: National Cancer Institute; 2013. Available online: https://seer.cancer.gov/, consulted online April 2019.
- Kane RA, Jacobs R, Katz J, et al. Porcelain gallbladder: ultrasound and CT appearance. Radiology 1984;152:137. [Crossref] [PubMed]
- Khan ZS, Livingston EH, Huerta S. Reassessing the need for prophylactic surgery in patients with porcelain gallbladder: case series and systematic review of the literature. Arch Surg 2011;146:1143-7. [Crossref] [PubMed]
- Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery 2001;129:699. [Crossref] [PubMed]
- Ashur H, Siegal B, Oland Y, et al. Calcified gallbladder (porcelain gallbladder). Arch Surg 1978;113:594. [Crossref] [PubMed]
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99. [PubMed]
- Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660. [Crossref] [PubMed]
- Sugiyama M, Atomi Y. Anomalous pancreaticobiliary junction without congenital choledochal cyst. Br J Surg 1998;85:911-6. [Crossref] [PubMed]
- Sugiyama M, Atomi Y. Endoscopic ultrasonography for diagnosing anaomalous pancreaticobiliary junction. Gastrointest Endosc 1997;45:261-7. [Crossref] [PubMed]
- Nagata E, Sakai K, Kinoshita H, et al. The relation between carcinoma of the gallbladder and anomalous correction between the choledochus and the pancreatic duct. Ann Surg 1985;202:182-90. [Crossref] [PubMed]
- Tanno S, Obara T, Fujii T, et al. Proliferative potential and K-ras mutation in epithelial hyperplasia of the gallbladder in patients with anomalous pancreaticobiliary duct union. Cancer 1998;83:267-75. [Crossref] [PubMed]
- Hanada K, Ito M, Fujii K, et al. K-ras, and p53 mutations in stage I gallbladder carcinoma with an anomalous junction of the pancreaticobiliary duct. Cancer 1996;77:452-8. [Crossref] [PubMed]
- Nagaraja V, Eslick GD. Systematic review with meta-analysis: the relationship between chronic Salmonella typhi carrier status and gall-bladder cancer. Aliment Pharmacol Ther 2014;39:745-50. [Crossref] [PubMed]
- Strom BL, Soloway RD, Rios-Dalenz JL, et al. Risk factors for gallbladder cancer. An international collaborative case control study. Cancer 1995;76:1747-56. [Crossref] [PubMed]
- Levy AD, Murakata LA, Rohrmann CA Jr. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics 2001;21:295-314; questionnaire, 549-55.
- Pandey M, Shukla VK. Lifestyle, parity, menstrual and reproductive factors and risk of gallbladder cancer. Eur J Cancer Prev 2003;12:269-72. [Crossref] [PubMed]
- Everson GT, McKinley C, Kern F Jr. Mechanisms of gallstone formation in women. Effects of exogenous estrogen (Premarin) and dietary cholesterol on hepatic lipid metabolism. J Clin Invest 1991;87:237-46. [Crossref] [PubMed]
- Wolin KY, Carson K, Colditz GA. Obesity and Cancer. Oncologist 2010;15:556-65. [Crossref] [PubMed]
- Lorenzo Bermejo J, Boekstegers F, Gonzalez Silos R, et al. Subtypes of Native American ancestry and leading causes of death: Mapuche ancestry-specific associations with gallbladder cancer risk in Chile. PLoS Genet 2017;13:e1006756. [Crossref] [PubMed]
- Tsuchiya Y, Terao M, Okano K, et al. Mutagenicity and mutagen of the red chili pepper as gallbladder cáncer risk factor in Chilean women. Asian Pac J Cancer Prev 2011;12:471-6. [PubMed]
- Asai T, Tsuchiya Y, Okano K, et al. Aflatoxin contamination of red chili pepper from Bolivia and Peru, countries with high gallbladder cancer incidence rates. Asian Pac J Cancer Prev 2012;13:5167-70. [Crossref] [PubMed]
- Nogueira L, Foerster C, Groopman J, et al. Association of aflatoxin with gallbladder cancer in Chile. JAMA 2015;313:2075-7. [Crossref] [PubMed]
- Koshiol J, Gao YT, Dean M, et al. Association of Aflatoxin and Gallbladder Cancer. Gastroenterology 2017;153:488-94.e1. [Crossref] [PubMed]
- Curado M, Edwards B, Shin H, et al. Cancer Incidence in Five Continents, Vol. IX. Lyon; IARC, 2007. Available online: http://ci5.iarc.fr/CI5I-X/old/vol9/CI5-IX_age-specific-tables.pdf, consulted June 2019.
- McGee EE, Jackson SS, Petrick JL, et al. Smoking, Alcohol, and Biliary Tract Cancer Risk: A Pooling Project of 26 Prospective Studies. Oxford University Press, 2019.
- Chan E, Berlin J. Biliary tract cancers: understudied and poorly understood. J Clin Oncol 2015;33:1845-8. [Crossref] [PubMed]
- Nemunaitis JM, Brown-Glabeman U, Soares H, et al. Gallbladder cancer: review of a rare orphan gastrointestinal cancer with a focus on populations of New Mexico. BMC Cancer 2018;18:665-79. [Crossref] [PubMed]
- Arroyo GF, Gentile A, Parada L. Gallbladder cancer: South American experience. Chin Clin Oncol 2016;5:67-80. [Crossref] [PubMed]
- Castillo J, Garcia P, Roa J. Alteraciones genéticas en lesiones preneoplásicas y neoplásicas de la vesícula biliar. Rev Med Chile 2010;138:595-604. [Crossref] [PubMed]
- Roa I, Araya J, Wistuba I, et al. Gallbladder cancer in the IX Region of Chile. Impact of the anatomopathological study of 474 cases. Rev Med Chil 1994;122:1248-56. [PubMed]
- Marcano-Bonilla L, Mohamed E, Mounajjed T, et al. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol 2016;5:61. [Crossref] [PubMed]
- Letelier P, Brebi P, Tapia O, et al. DNA promoter methylation as a diagnostic and therapeutic biomarker in gallbladder cancer. Clin Epigenetics 2012;4:11. [Crossref] [PubMed]
- Roa JC, Anabalón L, Roa I, et al. Promoter methylation profile in gallbladder cancer. J Gastroenterol 2006;41:269-75. [Crossref] [PubMed]
- Chang HJ, Jee C, Kim W. Mutation and altered expression of beta-catenin during gallbladder carcinogenesis. Am J Surg Pathol 2002;26:758-66. [Crossref] [PubMed]
- Sharma A, Lata K, Annapurna G, et al. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J Gastroenterol 2017;23:3978-98. [Crossref] [PubMed]
- Kanthan R, Senger J, Ahmed S, et al. Gallbladder cancer in the 21st century. J Oncol 2015;2015:967472. [Crossref] [PubMed]
- Yokoyama N, Hitomi J, Watanabe H, et al. Mutations of p53 in gallbladder carcinomas in high-incidence areas of Japan and Chile. Cancer Epidemiol Biomarkers Prev 1998;7:297-301. [PubMed]
- Wistuba II, Gazdar A, Roa I, et al. P53 protein overexpression in gallbladder carcinoma and its precursor lesions: an inmunohistochemical study. Hum Pathol 1996;27:360-5. [Crossref] [PubMed]
- Asai T, Loza E, Roig G, et al. High frequency of p53 but not K-ras gene mutations in Bolivian patients with gallbladder cancer. Asian Pac J Cancer Prev 2014;15:5449-54. [Crossref] [PubMed]
- Wistuba I, Gazdar A, Sugio K, et al. Abnormalities of p53 and K-ras gene in the pathogenesis of endemic gallbladder carcinoma (GBC) in Chile. Lab Invest 1995;72:70A. (abstract).
- Roa JC, Vo Q, Araya O, et al. Inactivation of CDKN2A gene (p16) in gallbladder carcinoma. Rev Med Chile 2004;132:1369-76. [PubMed]
- Akhoondi S, Sun D, von der Lehr N, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res 2007;67:9006-12. [Crossref] [PubMed]
- Arroyo G, Kaen D. F-box and WD repeat domain-containing 7 (FBXW7) mRNA and outcome in biliary tract cancer. J Clin Oncol 2012;30:e14521.
- Kim SW, Her K, Jang J, et al. K-ras oncogene mutation in cancer and precancerous lesions of gallbladder. J Surg Oncol 2000;75:246-51. [Crossref] [PubMed]
- Wistuba II, Sugio K, Hung J, et al. Allele-specific mutations involved in the pathogenesis of endemic gallbladder carcinoma in Chile. Cancer Res 1995;55:2511-5. [PubMed]
- Roa JC, Roa I, Aretxabala U, et al. K-ras gene mutation in gallbladder carcinoma. Rev Med Chile 2004;132:955-60. [PubMed]
- Roa JC, Anabalón L, Tapia O, et al. Frequency of K-ras mutation in biliary and pancreatic tumors. Rev Med Chile 2005;133:1434-40. [PubMed]
- Vidaurre T, Casavilca S, Montenegro P, et al. Tumor protein p53 and K-ras gene mutations in peruvian patients with gallbladder cancer. Asian Pac J Cancer Prev 2019;20:289-94. [Crossref] [PubMed]
- Kazmi HR, Chandra A, Nigam J, et al. Prognosis significance of K-ras codon 12 mutation in patients with resected gallbladder cancer. Dig Surg 2013;30:233-9. [Crossref] [PubMed]
- Kim YT, Kim J, Jang Y, et al. Genetic alterations in gallbladder adenoma, dysplasia and carcinoma. Cancer Lett 2001;169:59-68. [Crossref] [PubMed]
- Hanada K, Tsuchida A, Iwao T, et al. Gene mutations of K-ras in gallbladder mucosae and gallbladder carcinoma with an anomalous junction of the pancreaticobiliary duct. Am J Gastroenterol 1999;94:1638-42. [Crossref] [PubMed]
- Roa I, De Toro G, Schalper K, et al. Overexpression of the HER2/neu gene: a new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointest Cancer Res 2014;7:42-8. [PubMed]
- Arroyo G, Segovia R, Ituarte C, et al. Sobreexpresión de C-MET en carcinoma de vesícula y del tracto biliar: prevalencia y correlación clínico-molecular. Oncología Clínica 2017;22:77-84.
- Javle M, Bekaii-Saab T, Jain A, et al. Biliary Cancer: Utility of Next-Generation Sequencing for Clinical Management. Cancer 2016;122:3838-47. [Crossref] [PubMed]
- Arroyo G, Acosta G, Monteros Alvi M, et al. Kit expression in gallbladder cáncer. Proc Am Soc Clin Oncol 2003;22:303.
- Went PT, Dirnhofer S, Bundi M, et al. Prevalence of Kit expression in human tumors. J Clin Oncol 2004;22:4514-22. [Crossref] [PubMed]
- Aswad B, Constantinou M, Iannitti D, et al. Kit is a potential therapeutic target for biliary carcinomas. Proc Am Soc Clin Oncol 2002;21:103b.
- Zhang Z, Wang J, Shen B, et al. The ABCC4 gene is a promising target for pancreatic cancer therapy. Gene 2012;491:194-9. [Crossref] [PubMed]
- Ituarte C, Arroyo G, Sahores A, et al. Estudio de expresión y prevalencia del transportador MRP4/ABCC4 en cáncer de vesícula biliar y vías biliares en pacientes del noroeste argentino. Oral presentation (POP3) in: XXXVII Reunión de trabajos y actualización post Chicago. Buenos Aires 2018;5-6 de julio 2018. Argentina: Asociación Argentina de Oncología Clínica; 2018. Available online: http://aaoc.org.ar/cms/uploads/docs/postchicago-revista-2018.pdf
- Roa I, de Aretxabala X, Fuentealba P, et al. DNA content and survival in subserous gallbladder carcinoma. Rev Med Chile 2004;132:794-800. [PubMed]
- Takahashi T, Shivapurkar N, Riquelme E, et al. Aberrant promoter hypermethylation of multiple genes in gallbladder carcinoma and chronic cholecystitis. Clin Cancer Res 2004;10:6126-33. [Crossref] [PubMed]
- García P, Manterola C, Araya J, et al. Promoter methylation profile in preneoplastic and neoplastic gallbladder lesions. Mol Carcinog 2009;48:79-89. [Crossref] [PubMed]
- Roa JC, Roa I, Correa P, et al. Inestabilidad de microsatélite en lesiones preneoplásicas y neoplásicas de la vesícula biliar. J Gastroenterol 2005;40:79-86. [Crossref] [PubMed]
- Yanagisawa N, Mikami T, Yamashita K, et al. Microsatellite instability in chronic colecystitis is indicative of an early stage in gallbladder carcinogenesis. Am J Clin Pathol 2003;120:413-7. [Crossref] [PubMed]
- Campuzano Maya G. Utilidad clínica de los marcadores tumorales. Medicina & laboratorio 2010;16:411-45.
- Artico M, Bronzetti E, Alicino V, et al. Human Gallbladder Carcinoma: Role of Neurotrophins, MIB-1, CD34 and CA15-3. Eur J Histochem 2010;54:e10. [Crossref] [PubMed]
- Patel AH, Harnois D, Klee G, et al. The Utility of CA 19-9 in the Diagnoses of Cholangiocarcinoma in Patients without Primary Sclerosing Cholangitis. Am J Gastroenterol 2000;95:204-7. [Crossref] [PubMed]
- Chun Y, Pawlik T, Vauthey J. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 2018;25:845-7.
- de Aretxabala X, Roa I, Araya J, et al. Operative findings in patients with early forms of gallbladder cancer. Br J Surg 1990;77:291-3. [Crossref] [PubMed]
- Silva F, Alvarez C, Vergara J, et al. Cáncer de la vesícula biliar en colecistectomías en los hospitales del SSMS. Rev Chil Cir 2000;52:167-70.
- Manterola C, Vial M, Roa JC. Survival of a cohort of patients with Intermediate and advanced gall bladder cancer treated with a prospective therapeutic protocol. Acta Cir Bras 2010;25:225-30. [Crossref] [PubMed]
- Lendoire JC, Gil L, Duek F, et al. Relevance of residual disease after liver resection for incidental gallbladder cancer. HPB (Oxford) 2012;14:548-53. [Crossref] [PubMed]
- Chianale J, Valdivia G, del Pino G, et al. Gallbladder cancer mortality in Chile and its relation to cholecystectomy rates. An analysis of the last decade. Rev Med Chil 1990;118:1284-8. [PubMed]
- Alexander S, Lemmens V, Houterman S, et al. Gallbladder cancer, a vanishing disease? Cancer Causes Control 2012;23:1705-9. [Crossref] [PubMed]
- Diehl AK, Beral V. Cholecystectomy and changing mortality from gallbladder cancer. Lancet 1981;2:187-9. [Crossref] [PubMed]
- Ransohoff DF, Gracie W, Wolfenson L, et al. Prophylactic cholecystectomy of expectant management for silent gallstones. A decision analysis. Ann Intern Med 1983;99:199-204. [Crossref] [PubMed]
- Kottke TE, Feldman R, Albert D. The risk ratio is insufficient for clinical decision. The case of prophylactic cholecystectomy. Med Decis Making 1984;4:177-94. [Crossref] [PubMed]
- Ransohoff DF, Gracie WA. Treatment of gallstones. Ann Intern Med 1993;119:606-19. [Crossref] [PubMed]
- Noel R, Arnelo U, Lundell L. Does the Frequency of Cholecystectomy Affect the Ensuing Incidence of Gallbladder Cancer in Sweden? A Population-Based Study with a 16-Year Coverage. World J Surg 2016;40:66-72. [Crossref] [PubMed]
- Okamoto M, Okamoto H, Kitahara F, et al. Ultrasonographic evidence of association of polyps and stones with gallbladder cancer. Am J Gastroenterol 1999;94:446. [Crossref] [PubMed]
- Ministerio de Salud. Guía clínica. Colecistectomía preventiva en adultos de 35 a 49 años. Santiago de Chile: Minsal; 2010. Available online: https://www.minsal.cl/portal/url/item/72205a1420599f92e04001011f016d02.pdf, consulted online June 2019.
- Wibbenmeyer LA, Sharafuddin M, Wolverson M, et al. Sonographic diagnosis of unsuspected gallbladder cancer: imaging findings in comparison with benign gallbladder conditions. AJR Am J Roentgenol 1995;165:1169-74. [Crossref] [PubMed]
- Puschel K, Sullivan S, Montero J, et al. Análisis de costo-efectividad de un programa preventivo de enfermedad vesicular en Chile. Rev Med Chile 2002;130:447-9. [Crossref] [PubMed]
- Nervi F, Duarte I, Gómez G, et al. Frequency of gallbladder cancer in Chile, a high-risk area. Int J Cancer 1988;41:657-60. [Crossref] [PubMed]
- Kapoor VK. Cholecystectomy in patients with asymptomatic gallstones to prevent gall bladder cancer-the case against. Indian J Gastroenterol 2006;25:152-4. [PubMed]
- Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 1971;68:820. [Crossref] [PubMed]
- Pérez-Ayuso RM, Hernandez V, Gonzalez B, et al. Natural history of cholelithiasis and incidence of cholecystectomy in an urban and a Mapuche rural area. Rev Med Chil 2002;130:723-30. [Crossref] [PubMed]


