Emerging therapies for pancreas neuroendocrine cancers
Introduction
Neuroendocrine tumors (NET) are relatively rare malignancies, albeit with a rising incidence, which originate widely throughout the body. The incidence of NET has steadily increased in the last several decades, from 1.09/100,000 in 1974 to 5.25/100,000 in 2004, and the recent prevalence of NET was estimated to be 35/100,000 in the US population (1). These malignancies can arise in most organs (2), with the most common sites of NET origin being the gastrointestinal system followed by the lung/bronchus and the pancreas (3). Pancreas NET’s (PNET), or in older terminology, islet cell tumors, are usually considered as biologically more aggressive malignancies in comparison with NET from other sites and may produce more complex symptomatology related to hormone production depending on the cell of origin, e.g., insulinoma (insulin), glucagonoma (glucagon), VIPoma (vasoactive intestinal polypeptide), somatostatinoma (somatostatin), etc(4). Typically nonfunctional PNET’s are larger than the functional tumors and are usually non-hormone-producing (5) (Table 1).

Full table
Molecular biology
Blood vessel growth and formation (neoangiogenesis) and expansion play an important role in different vital pathological processes, especially in tumor growth. Vascular endothelial growth factor (VEGF) is the main growth factor responsible for angiogenesis by initiating the process of neovascularization and by interacting with specific transmembrane receptors that are expressed on the surface of endothelial cells (6). Several studies have described the overexpression of cellular growth factors and their receptors in NETs, including basic fibroblast growth factor (bFGF), VEGF, platelet derived growth factor (PDGF), insulin-like growth factor type 1 (IGF-1), epidermal growth factor (EGF), stem cell factor (KIT) and their related receptors (7). Based on these findings, several medications have been investigated to target this mechanism of action, including direct monoclonal antibody targeting VEGF, such as bevacizumab, and multiple tyrosine kinase inhibitors targeting VEGFR and other related receptors such as sunitinib, sorafenib and pazopanib (8).
The mammalian Target of Rapamycin (mTOR) is a serine-threonine kinase that has a role in cell growth, proliferation and apoptosis and also mediates downstream signaling in a number of pathways that are implicated in NETs growth, including VEGF, insulin receptor growth factor (IGF) and phosphoionositol 3-kinase (PI3K)-AKT signaling (6,9). Rarely PNET can arise in the setting of MEN-1, tuberosclerosis, von Hippel Landau (VHL) syndrome and neurofibromatosis, however the vast majority of PNET’s are sporadic, and studies have shown that gene alterations of MEN-1, DAXX/ATRX and mTOR pathway are common in sporadic PNET. Additionally the role of the PI3K/AKT/mTOR pathway in these types of tumors (10) supports the development of mTOR inhibitors as a treatment for PNET.
Pathology, classification and grading
Gross examination of a neuroendocrine malignancy can demonstrate a nodular, infiltrative or fungated mass, typically grayish or yellow to white in color; however these macroscopic feature are not specific and have no role in diagnosis (11). Typical microscopic histopathological features of NET’s include cells which have round or oval nuclei with chromatin and eosinophilic cytoplasm, and are usually arranged in trabecular or sheet-like pattern (12). Immunohistochemical studies are the best method to confirm the diagnosis of NETs. The most common stains include chromogranin and synatophysin. Other commonly used but less specific stains include, CDX2 (which may indicate small bowel origin), neuron specific endolase and CD56 (13).
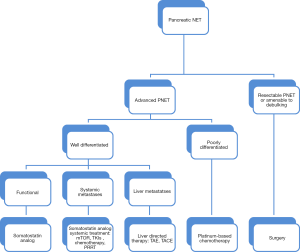
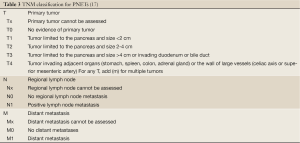
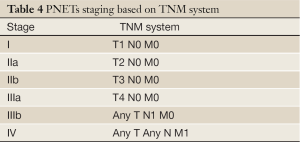
According to the 2010 WHO classification of GEP-NET, well-differentiated tumors are separated into low grade (G1) and intermediate grade (G2) categories, while all poorly differentiated NET are high grade (G3) (14) (Table 2). The grading system of NET depends mainly on the proliferation rate, which includes the mitotic activity and/or Ki-67 labeling index. Low grade (G1) includes mitotic counts of less than 2 per 2 mm2 and/or Ki-67 index of ≤2%; intermediate grade (G2) has mitotic count of 2 to 20 per 2 mm2 and/or Ki-67 index of 3-20%; and high grade (G3) shows mitotic count of ≥20 per 2 mm2 and/or Ki-67 ≥20% (12). Other pathologic characteristics such as lymphovascular and perineural invasion, and lymph node involvement are adjudicated as either positive or negative (15). A proposed TNM staging system for PNET may be helpful in the determination of patient’s prognosis and influences management plans (16) (Tables 3,4, Figure 1).

Full table
Full table
Diagnosis and imaging
Functional PNETs, especially insulinoma and gastrinoma can manifest hormonal symptoms even though the primary tumor may be very small (Table 1), and the localization of the primary lesion can be quite difficult (18). When a hormonal syndrome is identified, computed tomography (CT) or magnetic resonance imaging (MRI) are usually the initial imaging techniques to be used because of availability and speed. As NET’s are rich with blood vessels and radiologically hypervascular, the use of contrast, especially with multiphasic imaging (arterial and venous phases) is very helpful, while nonfunctioning PNET are most commonly found incidentally on CT or MRI when a patient is being evaluated for mass-related symptoms (19).
Conventional CT or MRI radiologic techniques usually detect about 70% of tumors larger than 3 cm, however they detect less than 50% of PNETs that are less than 1 cm in size, which leads to frequently missing primary PNETs and/or small liver metastases (20). As about 80% of PNETs express somatostatin receptors (SSTR), mainly SSTR-2 and SSTR-5, the use of somatostatin receptor scintigraphy (SRS) with indium111-labeled somatostatin analogue [(111In-DTPA0) octreotide] gives relatively high sensitivity and specificity as an imaging technique for PNETs and their metastases, although the resolution may be lower than CT or MRI. Several factors are limitations for somatostatin imaging, like the necessity of a background ratio of at least 2:1, and the low spatial resolution especially for small tumors, which have been overcome by the 68Ga-PET scan, and also a combination of positron emission tomography (PET)/CT which is able to provide additional anatomical information in respect to the localization and the boundaries of the lesions (4). Somatostatin scintigraphy has not been formally evaluated in the assessment of treatment response to therapy, and typically CT or MRI would be more helpful tests in such setting (21).
Endoscopic ultrasound (EUS) is a technique used for diagnosis and staging of many gastrointestinal tumors. The proximity of the echoscope to the pancreas offers a detailed view of the pancreas and an ability to identify small size PNETs, particularly insulinoma and gastrinomas, and can detect lesions as small as 2 to 5 mm. Thus EUS is quite sensitive in the detection of PNETs with a sensitivity of 82% and a specificity of 95% (22). EUS is also helpful in obtaining tissue confirmation for a diagnosis of PNET by EUS-guided fine needle aspiration (EUS-FNA). The aspirate is then evaluated by a cytopathologist, and a confirmed diagnosis usually determined by the immunohistochemical studies.
Treatment options
Somatostatin analogues
PNETs are characterized by high expression of somatostatin receptors (SSTR). Five different G-Protein-coupled SSRT subtypes have been recognized, SSRT 1 to 5. Among these, SSRT2 is expressed in almost 80% of PNET, and plays a major role in the management of this type of tumors (23).
Somatostatin is a 14-amino-acid peptide that inhibits the secretion of various hormones (including insulin, glucagon, growth hormone and gastrin) by binding to somatostatin receptors (24). Somatostatin analogues are a first line of treatment approved to control the symptoms resulting from functional PNET. Octreotide with its high affinity to SSRT-2 and less so to SSRT-5, was the first somatostatin analogue to be used clinically, when it was found to inhibit the secretion of growth hormone, glucagon and insulin more powerfully than somatostatin itself (25). Octreotide is a short-acting medication, which is given 2 to 3 times a day as a subcutaneous injection. Long-acting forms of somatostatin analogues were developed later, like octreotide LAR (long-acting-repeatable) and lanreotide with similar high affinity to SSRT-2 and SSRT-5. Octreotide LAR is given once every 4 weeks, while lanreotide is administrated every 2-4 weeks intramuscularly (26).
One of the first studies of a somatostatin analogue included 25 patients with NETs, treated with subcutaneous dosing of octreotide three times a day. Eighty-eight per cent of patients had control of symptoms (diarrhea and flushing), and 72% had a decrease in urinary 5-hydroxydoleacetic acid (5-HIAA) levels of 50% or more (27). It has been observed that somatostatin analogue administration resulted in tumor response in about 5-10% and stable disease in about 50% (23).
A prospective trial, the PROMID study, was designed as a placebo-controlled, double-blind, randomized study to evaluate the effect of octreotide LAR in the control of tumor growth in patients with metastatic midgut NET’s (28). Inclusion criteria included metastatic or locally inoperable disease, midgut primary tumor or tumor of unknown origin, well-differentiated NET by pathology, measurable disease by CT scan on MRI, more than 60% Karnofsky performance status and no curative treatment options available for the patient. Eighty-five patients were randomized to either octreotide 30 mg LAR (42 patients) or placebo (43 patients). The primary endpoint of time-to-tumor progression was 14.3 months in the octreotide group versus 6 months in the placebo group [95% confidence interval (CI), 0.20-0.59; P=0.000072]. The PROMID study secondary end points included overall survival, which was not reported in the initial study analysis, however was presented at the American Society of Clinical Oncology (ASCO) annual meeting in 2013. As of January 2013, 41 of the 85 patients enrolled had died, 19 in the octreotide group and 22 in the placebo group. The final results showed that octreotide LAR extends overall survival in patients with metastatic midgut NETs and a low hepatic tumor load (≤10% at study entry); (I) 10 patients in the octreotide group died versus 16 in the placebo group (total of 26 of 64 patients), while patient with an hepatic tumor load >10% had no survival benefits (29); (II) quality of life, was comparable in both groups, and (III) response rate which showed that stable disease was achieved in 66.7% in octreotide LAR group vs. 37.2% in placebo after 6 months of treatment (28).
In many countries, including the United States octreotide is not approved for asymptomatic or nonfunctional NETs, but it is frequently used as anti-neoplastic treatment to stop or slow the growth of metastatic disease (26). However, in the case of a negative somatostatin scintigraphy test, octreotide should not be administrated, especially in insulinoma where octreotide can prevent the compensatory effect of glucagon and lead to worsening hypoglycemia (21).
Peptide receptor radionucleotide therapy
The mechanism of action of peptide receptor radionucleotide therapy (PRRT) depends mainly on the overexpression of somatostatin receptors (SSTR) in NETs over the surrounding normal tissue. PRRT can be used in patients with unresectable PNET, with or without metastases and expressing high level of SSTRs. Radiopeptides bind to the SSRTs on the cell surface and are internalized to deliver localized radiotherapy to the tumor cell with little effect on surrounding tissue. The most frequently used radionuclides for targeted radiotherapy include yttrium (90Y), lutetium (177Lu) or indium (111In) linked to a somatostatin analogue. The greater the expression of SSRTs by the tumor cell potentially the more effective PRRT may be and thus an octreotide or gallium scan can be useful to predict the potential benefits of the PRRT (the higher uptake, the more effect) (7).
In a single center analysis from the University Hospital Basel, Basel, Switzerland, 1,109 patients with metastatic NETs were treated between October 1997 and February 2010, and received somatostatin-based radiopeptide therapy with 90yttrium-labeled tetraazacyclododecane-tetraacetic acid modified Tyr3-octreotide (90Y-DOTATOC) (30). Of the 1,109 patients, 378 (34.1%) had morphological response, 172 (15.5%) had biochemical response and 329 (29.7%) had clinical response. Tumor response was associated with longer survival compared to disease progression. Adverse events included grade 3 and 4 transient hematological toxicity in 142 patients (12.8%), including leucopenia, anemia and thrombocytopenia. Two patients developed myeloproliferative disease, two patients developed tumor lysis syndrome with reversible renal failure, and 102 (9.2%) patients developed severe permanent renal toxicity (grade 4 and 5) (30).
In another study, a single-arm multicenter review that included patients from five European countries and the United States, 90 patients with NETs with symptoms refractory to octreotide were enrolled between July 2001 and August 2002 with last follow up in 2004. All 90 patients received 90Y-edotreotide (31), and in this series 4 patients (4.4%; 95% CI, 0.2% to 8.6%) had a partial response, and 63 (70%) had stable disease. Of the 90 patients, 54 (60%) had grade 3 to 4 adverse events mainly as lymphopenia, nausea and vomiting, and three patients (3.3%) had grade 3 to 4 renal toxicity, which was transient (31). The main gastrointestinal side effects including nausea and vomiting were likely related to the co-infusion of amino acids that were administered as organ protectants (32).
PRRT has been largely used in Europe. It has limited availability in the United States for several reasons, including the absence of controlled, randomized multicenter clinical trials, along with the restricted access to several radionuclide peptides due to the regulations in the U.S., otherwise PRRT might be more frequently considered as a first line management strategy in unresectable somatostatin-positive disease (33).
Targeted therapies
Multi-targeted tyrosine kinase inhibitors and anti-angiogenic agents, e.g., Sunitinib, have activity in inhibiting all types of VEGFR and other tyrosine kinase receptors (6). One of the initial clinical trials in NET’s evaluating sunitinib was a phase II study in NET’s in 107 patients who received sunitinib at a dose of 50 mg daily for 4 weeks of every 6 weeks. Of the 66 patients with advanced PNETs, 11 (16.7%) had a partial response and 45 patients (68%) had stable disease (34).
A key phase III, multi-national, randomized, double-blinded, placebo-controlled study, compared sunitinib (37.5 mg daily) with placebo in 171 patients with progressive PNET (35). Patients were recruited between June 2007 and April 2009. The study was discontinued because of the difference in the progression-free survival favoring the sunitinib group and the greater number of deaths in the placebo group. Progression-free survival, the primary endpoint, in the sunitinib group was 11.4 versus 5.5 months in the placebo group (95% CI, 0.26 to 0.66; P<0.001). The objective tumor response rate, a secondary end point, was 9.3% in the sunitinib group versus 0% in the placebo group (35). Based on this study, the US Food and Drug Administration (FDA) approved sunitinib in May 2011 for the treatment of advanced PNET and it was also approved few months later in Europe and other countries around the world.
A recent small multicenter, phase II trial evaluated sunitinib in Japanese patients with well-differentiated PNET. Twelve patients received sunitinib dosed at 37.5 mg daily, of which six patients had a partial response and three had stable disease, for a clinical benefit response rate of 75% (95% CI, 43-94%). Progression-free survival was 91% (95% CI, 54-99%) at 6 months and 71% (95% CI, 34-90%) at 12 months (36).
Sunitinib has become integrated into the treatment of progressive well-differentiated pancreatic NET. The most common grade 1 and 2 side effects associated with sunitinib from the phase III experience are, diarrhea, nausea, asthenia, vomiting and fatigue, each occurring in more than 30% of patients. Other side effects include hand-foot syndrome (in about 23%), while grade 3 and 4 adverse effects included neutropenia (12%) and hypertension (10%) (35).
Sorafenib and pazopanib are other tyrosine kinase inhibitors, which have been evaluated in NET’s. Sorafenib targets VEGFR-2, PDGFR-beta and raf kinase, and was initially evaluated in the treatment of advanced renal cell carcinoma and hepatocellular carcinoma (37). In a phase II trial of sorafenib in NET’s, 93 patients were enrolled, 43 with PNET and 50 with carcinoid tumors. Ten percent of the patients (8 patients) had a partial response and 12.9% (12 patients) had a minor response (20-29% decrease in target lesion) for an overall response rate of 32% in PNET and 17% in carcinoid tumors. Six months progression-free survival was 60.8% in the PNET group and 40% in carcinoid tumors for evaluable patients (38).
A phase II trial evaluated the combination of sorafenib and bevacizumab in patients with advanced NET. Forty-four patients were enrolled and the overall response rate was 9.8% and the disease control rate was 95.1%. The estimated progression-free survival was 12.4 months (95% CI: 9.4-16.2 months) (39).
Pazopanib, which also inhibits both VEGFR and PDGFR, has been assessed in a phase II clinical trial in which 51 patients with NET on stable octreotide LAR doses were treated with pazopanib. Of the 46 patients who completed 12 weeks of treatment and were evaluable for response, 30 patients had PNET and the response rate was 17% (5/30). The progression-free survival rate was 80% after 24 weeks, and median PFS times were 11.7 months in PNET patients (40).
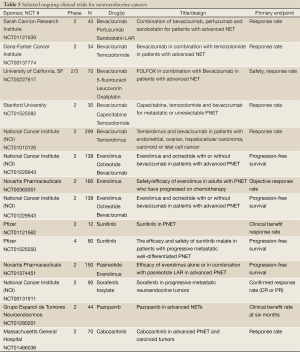
Bevacizumab, a monoclonal antibody that binds to VEGF in the blood and inhibits the binding of VEGF to its receptors in the endothelial cells, and thus inhibiting angiogenesis (41). Forty-four patients on a stable dose of octreotide were enrolled in a phase II clinical trial to receive 18 weeks of either bevacizumab or pegylated-interferon alpha-2b. At the end of 18 weeks or progression of disease (whichever first) patients received the combination of both therapies along with continued octreotide. The results revealed that only 5% of patients in the bevacizumab group had progression of disease while 27% of the pegylated-interferon alpha-2b group had progression of disease, and the corresponding progression-free survivals were 95% in the bevacizumab versus 68% in the pegylated interferon alpha-2b group after 18 weeks of monotherapy (42). There are several ongoing trials investigating the efficacy and safety of bevacizumab in combination with other agents in the treatment of PNETs (Table 5).
Full table
Overall, the collective experience evaluating anti-angiogenic and tyrosine kinase inhibitors in NET’s and PNET’s in particular, has yielded encouraging results with one approved drug in PNET’s (sunitinib) and several others in late stage development.
mTOR inhibitors
mTOR is a serine-threonine kinase that regulates cell growth and proliferation. Several cancer-promoter ligands such as EGFR, AKT, PI3K, HER2 and BCR-ABL, can activate mTOR and stimulate cell growth, proliferation and survival. The mTOR signaling pathway is one of the major pathways that are found to be dysregulated in many neoplasms and play a big role in cancer growth and pathogenesis (43).
Everolimus is an mTOR inhibitor which is orally bioavailable and which binds to its intracellular receptor with high affinity and interacts with mTOR to inhibit the downstream signaling pathway and prevent cancer cell proliferation (44). Everolimus was initially evaluated for NETs in a phase II study in combination with octreotide LAR (45). Sixty patients were enrolled and received 5 or 10 mg of everolimus daily in combination with octreotide LAR. Twenty-two per cent of patients had a partial response and 70% had stable disease and the median progression-free survival was 60 weeks (45).
A phase III, international, multicenter, double-blind, randomized trial (RADIANT-3) assigned 410 patients with advanced low grade or intermediate grade PNET from 18 countries between July 2007 and May 2009, to receive everolimus 10 mg daily or placebo along with best supportive care (46). Eligibility criteria included adults with low-grade or intermediate-grade advanced PNET with disease progression in the last 12 months. Patients were excluded if they had prior treatment with mTOR inhibitor, had hepatic artery embolization in the last 6 months, cryoablation or radiofrequency ablation in the last 2 months or were receiving long-term treatment with immunosuppressants. The primary endpoint, median PFS, was 11 months (95% CI, 8.4 to 13.9 months) for patients treated with everolimus versus 4.6 months for patients treated with placebo (95% CI, 3.1 to 5.4 months). The percentage of patients with PFS at 18 months was 34% (95% CI, 26% to 43%) in the everolimus group versus 9% (95% CI, 4% to 16%) in the placebo group (46). Grade 1 and 2 side effects included stomatitis (64%), rash (49%), diarrhea (34%), fatigue (31%), infection (23%) and pneumonitis (12%), while grade 3 and 4 side effects included hyperglycemia (5%), anemia (6%), stomatitis (7%), and thrombocytopenia (4%) (46).
Other evaluations of everolimus have been undertaken, e.g., in a phase II clinical trial where everolimus was given in combination with bevacizumab. Results showed an overall response rate of 26% and stable disease in 69% of patients. The progression-free survival rate at 6-month was 92% and the median progression-free survival was 14.4 months (95% CI, 12.7-16.1 months) (47). These data suggest that the combination of octreotide and everolimus may have clinical utility.
Temsirolimus, another mTOR inhibitor, which is given intravenously, has been evaluated as monotherapy in a phase II study that enrolled 37 patients to receive temsirolimus 25 mg intravenously weekly. The objective response rate was 5.6% (95% CI, 0.6-18.7%) while 63.9% (95% CI, 46.2-79.2%) of patients had disease control (stable disease or partial response), with a reported survival rate of 91.6% (95% CI, 82.9-100%) at 6 months and 71.5% (95% CI, 57.1-89.5%) at 1-year (48). Further evaluation of temsirolimus (25 mg intravenously weekly) in combination with bevacizumab (10 mg/kg every two weeks) was studied in a phase II clinical trial for which preliminary results were presented at the 2012 Gastrointestinal Cancers symposium (49). Data from 35 patients with progressive PNET had a partial response in 52% of patients and the 6-month progression-free survival was 84% in evaluable patients (49). The major side effects of this treatment combination were hypertension, leucopenia, lymphopenia, hyperglycemia, mucositis, hypokalemia and fatigue (49). Table 5 summarizes trials that are ongoing evaluating targeted therapies in NET’s.
Cytotoxic therapies
Pancreatic NET’s typically can respond to cytotoxic chemotherapy, with traditionally alkylating agents being utilized. Streptozocin-based treatment was one of the early cytotoxic regimens to be evaluated in patients with advanced PNET. In a multicenter randomized trial, streptozocin plus doxorubicin was demonstrated to be superior to doxorubicin plus 5-fluorouracil in regard to response rate (69% vs. 45%, P=0.05) and median survival (2.2 vs. 1.4 years, P=0.04) (50). A large retrospective study evaluated 84 patients with either locally advanced or metastatic NET that received streptozocin, 5-FU and doxorubicin. Activity was demonstrated with a response rate of 39% (95% CI, 27% to 50%), median progression-free survival of 18 months and 2-year progression-free survival was 41% (95% CI, 26% to 56%). The median overall survival was 37 months and the 2-year overall survival was 74% (95% CI, 61% to 87%) (51).
Temozolomide, another alkylating agent with activity against NET, was evaluated as a monotherapy in a retrospective study of 36 patients. A partial response was observed in 14% of patients and stable disease in 53%. The median overall time to progression was 7 months (95% CI, 3 to 10 months) (52). Subsequently, temozolomide was given in combination with other agents. In one study 34 patients were treated with temozolomide combined with bevacizumab. The response rate in PNET patients was 33%, and the median progression-free survival was 14.3 months and median overall survival was 41.7 months (53). A further trial evaluated the combination of temozolomide with everolimus in a phase I/II clinical trial of 24 patients with advanced PNET. Thirty-five per cent had a partial response and 53% had stable disease (54).
Emerging data has demonstrated the utility of temozolomide combined with capecitabine in NET’s. Specifically in a retrospective study of 30 patients with metastatic PNET treated with temozolomide in combination with capecitabine, an overall response rate of 70% (95% CI, 54-86%) was observed and the overall survival at two years was 92% (95% CI, 72-98%) and the median progression free survival was 18 months (95% CI, 9-31 months) (55). An ongoing phase II clinical trial is being conducted to evaluate the efficacy and safety of temozolomide and capecitabine combination in progressive differentiated NETs (NCT00869050). A planned phase II study by the Eastern Cooperative Oncology Group (ECOG) and National Cancer Institution (NCI) will randomize patients to temozolomide with or without capecitabine to evaluate the survival and response rate in advanced PNET (NCT01824875).
Alkylating agents, such as temozolomide, have the ability to induce cell death and apoptosis by the methylation of DNA at the O6 position of guanine. Subsequently the methylation of guanine will lead to DNA mismatch and apoptosis of tumor cells (56). A DNA repair enzyme, O6-methylguanine-DNA methyltransferase (MGMT), has the ability to restore the damaged DNA to its original form and prevent cell death, and so the availability of this enzyme will determine the sensitivity of tumor cells to alkylating agents (57), in other words, the deficiency of MGMT enzyme is associated with better response to temozolomide-based treatment and better survival, while the availability of this enzyme can contribute to resistance to temozolomide as well as other alkylating drugs such as streptozocin and dacarbazine (56).
Oxaliplatin-containing regimens have recently been evaluated in NET’s. A phase II trial of FOLFOX (oxaliplatin, 5-fluorouracil and leucovorin) and bevacizumab revealed a partial response in 33% and stable disease in 67% of patients with pancreatic NETs (58). Another phase II trial assessing the activity of oxaliplatin in combination with capecitabine and bevacizumab in patients with advanced NETs reported 23% of the patients as having a partial response and 71% had stable disease. The one-year progression-free survival was 52% and the median progression-free survival was 13.7 months (59).
Poorly differentiated PNETs tend to be more responsive to cytotoxic chemotherapy than well-differentiated PNET’s. For these former patients, particularly the high grade poorly differentiated PNET’s cytotoxic therapy is likely the mainstay with treatment options including cisplatin and etoposide, streptozotocin-based therapies, and emerging options of capecitabine and temozolomide and oxaliplatin/fluoropyrimidine and bevacizumab combinations. One study of cisplatin and etoposide, a commonly used combination regimen for small cell lung cancer, was used to treat 45 patients with neuroendocrine carcinoma (60). The treatment was associated with a 67% response rate in the 18 patients with poorly differentiated NETs, while it had little effect on well-differentiated NETs. In another study, 36 patients with advanced NET (poorly differentiated or rapidly progressing NET) were treated with cisplatin and etoposide. The overall response rate for PNET was 36% and median survival time was 13 months (61). In a phase II prospective study, 78 patients with poorly differentiated NETs (exact type not specified) were treated with carboplatin, etoposide and paclitaxel. The response rate was 53% and the overall survival was 14.5 months (95% CI, 9.5 to 18.5 months). However this three-drug regimen was moderately toxic and did not have an efficacy advantage when compared to the standard platinum plus etoposide regimens (62).
To summarize, cytotoxic therapy has an established mainstay for the treatment of PNET’s, albeit with few randomized trials to guide therapy. Specifically, for high grade poorly differentiated PNET’s, a platinum and etoposide combination remains a reference standard. For well-intermediate differentiated PNET’s, capecitabine and temozolomide is an emerging standard and it remains to be seen whether routine evaluation of MGMT promoter methylation status will be validated for refining therapy.
Regional and liver directed therapies
Cytoreductive surgery, or debulking surgery, defined as the removal of >90% of the tumor volume including the primary tumor if present, is usually conducted in functional NETs to reduce symptoms when medical treatment has been maximized, and this type of intervention is occasionally justified as a palliative measure (63). Debulking surgery typically refers to debulking of hepatic metastases when the liver is the predominant site of spread with mainly intrahepatic tumor growth, slowly growing tumors and in settings when the primary tumor and its metastases are amenable for resection (64). As reported in the literature in retrospective series of highly selected patients, a significant percentage of patients, 88%, can achieve effective symptom control and the 5-year survival rate approaches 60-70% after debulking surgery (64).
Hepatic artery embolization (HAE) is considered in patients with liver predominant disease and disease typically not amenable for surgical resection. Studies have shown HAE to be effective in slowing tumor growth and alleviating symptoms caused by the tumor. Hepatic artery embolization can be performed with bland trans-catheter arterial embolization (TAE), trans-catheter arterial chemoembolization (TACE) or chemoembolization with drug-eluting beads (65). At this time, there is no consensus as to which modality is preferable. The chemotherapy agent used in chemoembolization (TACE) is usually doxorubicin or streptozocin in combination with lipiodol, and the response rate in one series was 67% of the treated patients (66). Side effects can develop after the embolization due to post-embolization syndrome such as nausea and vomiting (50-70%), upper quadrant abdominal pain (50-60%), fever (30-60%), and transaminitis (100%), all of which are usually mild and transient, while major complications are rare but can include liver failure, renal failure, bleeding, infection (higher risk following prior biliary reconstruction) and even death. The relative to absolute contraindications to this type of treatment include, complete portal vein thrombosis, hepatic failure and previous biliary anastomosis (67), where the latter can dramatically increase the risk of liver abscesses, which in one series occurred in up to 33% of such patients (68).
Radioembolization is another embolization technique that is gaining traction in the treatment of NET’s and uses radioactive isotopes such as yttrium 90 (90Y). These isotopes are infused through a catheter via the hepatic artery to reach tumor arteriolar level where high dose radiation is delivered and results in tumor necrosis (69). In several studies, the response rate was about 42-63% (69-71). Grade 1 and 2 toxicities included fatigue (56%), abdominal pain (26%), nausea (23%) and fever (6%) (69). Grade 3 toxicities were fatigue (6.5%), nausea (3.2%) and pain (2.7%) (71).
There are no randomized studies to compare among TAE, TACE or radioembolization; thus, any one of these three techniques is considered an appropriate palliative treatment for patients with NET with liver metastases. However, the field of locoregional therapies for NET’s would greatly benefit from carefully designed prospective randomized trials to better understand the oncologic impact, selection criteria and other considerations which would identify one approach as being superior to another for selected subgroups of patients.
Conclusions
Significant progress has been made in understanding the changing epidemiology and molecular biology and classification of PNET’s. Treatment options are varied and include surgery, regional approaches, targeted therapies and systemic cytotoxic therapies. The broad range of therapeutic considerations requires careful multi-disciplinary assessment to determine which approach is best for a given patient at a given time point in the disease course and this point underscores an urgent need in the field to study sequencing of therapies and combinations of approaches.
Systemic treatment options for pancreatic neuroendocrine tumors include somatostatin analogues as first line treatment if patients have somatostatin receptors positive tumors, and are recommended for control of hormonal production and for anti-proliferative effects in low grade well-differentiated PNET’s. Cytotoxic combination therapy such as temozolomide and capecitabine is an emerging combination and may be an initial step for a subset of patients with symptomatic, SSR negative, intermediate to high grade, or progressing disease. Several recent studies have demonstrated that tyrosine kinase inhibitors such as sunitinib and mTOR inhibitors such as everolimus both improve progression-free survival in patients with advanced well-differentiated PNETs and both have become established regulatory authority approved therapies. In poorly differentiated pancreatic NET with very high mitotic rates (e.g., small cell type), combination systemic chemotherapy with platinum-based therapies similar to the regimens used for small cell lung carcinoma, are typically considered as a first step. For poorly differentiated, grade 3 NET’s with mitotic rates >20% but not with proliferative rates akin to small cell type NET’s, there is no consensus as to what is the best initial therapy. Regionally directed therapies with bland embolization, TACE or radioembolization may alleviate symptoms and give palliation for patients with liver confined or predominant disease. Peptide receptor radionucleotide therapy (PRRT) is a treatment modality that has shown significant promise but requires further rigorous investigation with carefully designed dosimetry studies to determine the optimal dose of the radio-isotope and to more fully evaluate the short and longer-term side effects along with evaluation as to when in the sequence of the range of available therapies for NET’s it should best fit.
To summarize, PNET’s traditionally a disease with limited treatment options, has become an entity with several newly approved therapies and for which investigators and the pharmaceutical industry alike have a newfound interest in drug development, research and clinical trials. The next decade of research is bright in PNET’s and should provide new insights into the molecular underpinnings of this disease, therapy selection and sequencing of the available therapies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35, 825 cases in the United States. J Clin Oncol 2008;26:3063-72. [PubMed]
- Öberg K, Knigge U, Kwekkeboom D, et al. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii124-30. [PubMed]
- Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg 2004;240:117-22. [PubMed]
- Frilling A, Akerström G, Falconi M, et al. Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer 2012;19:R163-85. [PubMed]
- Kazanjian KK, Reber HA, Hines OJ. Resection of pancreatic neuroendocrine tumors: results of 70 cases. Arch Surg 2006;141:765-9; discussion 769-70. [PubMed]
- Naraev BG, Strosberg JR, Halfdanarson TR. Current status and perspectives of targeted therapy in well-differentiated neuroendocrine tumors. Oncology 2012;83:117-27. [PubMed]
- Demirkan BH, Eriksson B. Systemic treatment of neuroendocrine tumors with hepatic metastases. Turk J Gastroenterol 2012;23:427-37. [PubMed]
- De Dosso S, Grande E, Barriuso J, et al. The targeted therapy revolution in neuroendocrine tumors: in search of biomarkers for patient selection and response evaluation. Cancer Metastasis Rev 2013. [Epub ahead of print]. [PubMed]
- Missiaglia E, Dalai I, Barbi S, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol 2010;28:245-55. [PubMed]
- Bergsland EK. The evolving landscape of neuroendocrine tumors. Semin Oncol 2013;40:4-22. [PubMed]
- Stoica-Mustafa E, Pechianu C, Iorgescu A, et al. Pathological characteristics and clinical specifications in gastroenteropancreatic neuroendocrine tumors: a study of 68 cases. Rom J Morphol Embryol 2012;53:351-5. [PubMed]
- Hirabayashi K, Zamboni G, Nishi T, et al. Histopathology of gastrointestinal neuroendocrine neoplasms. Front Oncol 2013;3:2. [PubMed]
- Kim MK. Endoscopic ultrasound in gastroenteropancreatic neuroendocrine tumors. Gut Liver 2012;6:405-10. [PubMed]
- Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707-12. [PubMed]
- Cho MY, Kim JM, Sohn JH, et al. Current trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea 2000-2009: multicenter study. Cancer Res Treat 2012;44:157-65. [PubMed]
- Pape UF, Jann H, Müller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 2008;113:256-65. [PubMed]
- Rindi G, Klöppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006;449:395-401. [PubMed]
- Kulke MH, Bendell J, Kvols L, et al. Evolving diagnostic and treatment strategies for pancreatic neuroendocrine tumors. J Hematol Oncol 2011;4:29. [PubMed]
- Halperin DM, Kulke MH. Management of pancreatic neuroendocrine tumors. Gastroenterol Clin North Am 2012;41:119-31. [PubMed]
- Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010;39:735-52. [PubMed]
- Reidy DL, Tang LH, Saltz LB. Treatment of advanced disease in patients with well-differentiated neuroendocrine tumors. Nat Clin Pract Oncol 2009;6:143-52. [PubMed]
- Rösch T, Lightdale CJ, Botet JF, et al. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med 1992;326:1721-6. [PubMed]
- Toumpanakis C, Caplin ME. Update on the role of somatostatin analogs for the treatment of patients with gastroenteropancreatic neuroendocrine tumors. Semin Oncol 2013;40:56-68. [PubMed]
- Kulke MH, Mayer RJ. Carcinoid Tumors. N Engl J Med 1999;340:858-68. [PubMed]
- Lamberts SW, van der Lely AJ, de Herder WW, et al. Octreotide. N Engl J Med 1996;334:246-54. [PubMed]
- Wolin EM. The expanding role of somatostatin analogs in the management of neuroendocrine tumors. Gastrointest Cancer Res 2012;5:161-8. [PubMed]
- Kvols LK, Moertel CG, O’Connell MJ, et al. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med 1986;315:663-6. [PubMed]
- Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. [PubMed]
- Arnold R, Wittenberg M, Rinke A, et al. J Clin Oncol 2013;31:abstr 4030. 2013 ASCO Annual Meeting.
- Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29:2416-23. [PubMed]
- Bushnell DL Jr, O’Dorisio TM, O’Dorisio MS, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol 2010;28:1652-9. [PubMed]
- Cwikla JB, Sankowski A, Seklecka N, et al. Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): a phase II study. Ann Oncol 2010;21:787-94. [PubMed]
- Graham MM, Menda Y. Radiopeptide imaging and therapy in the United States. J Nucl Med 2011;52 Suppl 2:56S-63S. [PubMed]
- Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 2008;26:3403-10. [PubMed]
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [PubMed]
- Ito T, Okusaka T, Nishida T, et al. Phase II study of sunitinib in Japanese patients with unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumor. Invest New Drugs 2013;31:1265-74. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Hobday TJ, Rubin J, Holen K, et al. MC044h, a phase II trial of sorafenib in patients (pts) with metastatic neuroendocrine tumors (NET): A Phase II Consortium (P2C) study. J Clin Oncol 2007;25:abstr 4504.
- Castellano DE, Capdevila J, Salazar R, et al. Sorafenib and bevacizumab combination targeted therapy in advanced neuroendocrine tumor: A phase II study of the Spanish Neuroendocrine Tumor Group (GETNE0801). Journal of Clinical Oncology 2011 ASCO Annual Meeting Proceedings (Post-Meeting Edition),2011:29.
- Phan AT, Yao JC, Fogelman DR, et al. A prospective, multi-institutional phase II study of GW786034 (pazopanib) and depot octreotide (sandostatin LAR) in advanced low-grade neuroendocrine carcinoma (LGNEC). J Clin Oncol 2012;28:abstr 4001.
- Kasuya K, Nagakawa Y, Suzuki M, et al. Anti-vascular endothelial growth factor antibody single therapy for pancreatic neuroendocrine carcinoma exhibits a marked tumor growth-inhibitory effect. Exp Ther Med 2011;2:1047-52. [PubMed]
- Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol 2008;26:1316-23. [PubMed]
- Zhang YJ, Duan Y, Zheng XF. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today 2011;16:325-31. [PubMed]
- O’Donnell A, Faivre S, Burris HA 3rd, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 2008;26:1588-95. [PubMed]
- Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors:results of a phase II study. J Clin Oncol 2008;26:4311-8. [PubMed]
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. [PubMed]
- Yao JC, Phan AT, Fogleman D, et al. Randomized run-in study of bevacizumab and everolimus in low- to intermediate-grade neuroendocrine tumors using perfusion CT as functional biomarker. 2010 ASCO Annual Meeting, J Clin Oncol 2012;28:abstr 4002.
- Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer 2006;95:1148-54. [PubMed]
- Hobday TJ, Qin R, Reidy DL, et al. Multicenter phase II trial of temsirolimus and bevacizumab in pancreatic neuroendocrine tumor: Results of a planned interim efficacy analysis. J Clin Oncol 2012;30:abstr 4047.
- Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992;326:519-23. [PubMed]
- Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762-71. [PubMed]
- Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res 2007;13:2986-91. [PubMed]
- Chan JA, Stuart K, Earle CC, et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol 2012;30:2963-8. [PubMed]
- Kulke M, Blaszkowsky LS, Zhu AX, et al. Phase I/II study of everolimus (RAD001) in combination with temozolomide in patients with advanced pancreatic neuroendocrine tumors. 2010 ASCO Gastrointestinal Cancers Symposium. Abstract No. 223.
- Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268-75. [PubMed]
- Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res 2009;15:338-45. [PubMed]
- Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol 2011;29:934-43. [PubMed]
- Venook AP, Ko AH, Tempero MA, et al. Phase II trial of FOLFOX plus bevacizumab in advanced, progressive neuroendocrine tumors. J Clin Oncol 2008;26:abstr 15545.
- Kunz PL, Kuo T, Kaiser HL, et al. A phase II study of capecitabine, oxaliplatin, and bevacizumab for metastatic or unresectable neuroendocrine tumors: Preliminary results. J Clin Oncol 2008;26;abstr 15502.
- Moertel CG, Kvols LK, O’Connell MJ, et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991;68:227-32. [PubMed]
- Fjällskog ML, Granberg DP, Welin SL, et al. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer 2001;92:1101-7. [PubMed]
- Hainsworth JD, Spigel DR, Litchy S, et al. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuroendocrine carcinoma: a Minnie Pearl Cancer Research Network Study. J Clin Oncol 2006;24:3548-54. [PubMed]
- Steinmüller T, Kianmanesh R, Falconi M, et al. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2008;87:47-62. [PubMed]
- Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin N Am 2003;12:231-42. [PubMed]
- Mayo SC, Herman JM, Cosgrove D, et al. Emerging approaches in the management of patients with neuroendocrine liver metastasis: role of liver-directed and systemic therapies. J Am Coll Surg 2013;216:123-34. [PubMed]
- Gupta S, Yao JC, Ahrar K, et al. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: the M.D. Anderson experience. Cancer J 2003;9:261-7. [PubMed]
- O’Toole D, Maire F, Ruszniewski P. Ablative therapies for liver metastases of digestive endocrine tumours. Endocr Relat Cancer 2003;10:463-8. [PubMed]
- Mezhir JJ, Fong Y, Fleischer D, et al. Pyogenic abscess after hepatic artery embolization: a rare but potentially lethal complication. J Vasc Interv Radiol 2011;22:177-82. [PubMed]
- Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres--safety, efficacy, and survival. Radiology 2008;247:507-15. [PubMed]
- King J, Quinn R, Glenn DM, et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer 2008;113:921-9. [PubMed]
- Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol 2008;31:271-9. [PubMed]