
Contemporary perspectives on the use of radiation therapy for locally advanced gallbladder cancer
Introduction
Although gallbladder cancer is relatively uncommon, it is the most common biliary tract cancer and is associated with a highly aggressive natural history. Owing to the low incidence of gallbladder cancer, well-powered randomized clinical trials have not been possible, and definitive evidence-based approaches to management are lacking in the literature. As a result, management decisions of gallbladder cancer continue to be centered on multidisciplinary discussion, carefully weighing the risks and benefits of various combinations of surgical resection, chemotherapy, and radiation therapy.
Although survival for gallbladder cancer remains poor, surgical resection remains the only recognized curative approach (1). However, many patients may not be candidates for resection owing to the extent and/or spread of disease. Chemotherapy is considered an integral portion of therapy in the post-operative or definitive setting, but does not substitute for definitive local therapy. The use of RT is controversial, yet is commonly used for selected cases in the adjuvant or definitive setting. The National Comprehensive Cancer Network (NCCN) currently recommends up-front resection when possible, followed by adjuvant chemotherapy or chemotherapy plus radiation therapy; if resection is not feasible, definitive chemotherapy plus radiation therapy or chemotherapy alone are also options (2).
The aim of this review is to critically examine the role of radiation therapy for gallbladder cancer, both in the adjuvant and definitive settings, as well as describe novel RT approaches and treatment paradigms of the contemporary era. The reader is cautioned that the overall quality and quantity of data are relatively limited, and hence this article does not substitute for careful, individualized judgment as part of a multidisciplinary team approach.
Rationale
Patterns-of-failure studies demonstrate the propensity for gallbladder cancer to metastasize, the high frequency locoregional recurrence after surgery alone, and a poor overall survival (OS) rates in patients with unresectable disease. Gallbladder cancer is a distinct entity from other biliary tract cancers. In comparison, patients with intrahepatic cholangiocarcinoma have a high distant metastatic rate; in spite of this, local disease progression leads to mortality in the vast majority of cases if a definitive local treatment is not possible (3,4). Patients with inoperable extrahepatic cholangiocarcinoma suffer significant morbidly and mortality from their primary disease and typically die from biliary sclerosis related to relentless bouts of cholangitis. There is also a very high locoregional recurrence rate after resection owing to the high incidence of close and positive margins and the propensity for isolated nodal spread (5,6), which supports the use of local therapy following resection.
In contrast, following resection of gallbladder cancer an estimated 15–25% of recurrences occur as locoregional recurrences if radiation therapy or chemotherapy plus radiation therapy is not used (7-12) (Table 1). Of note, these recurrence patterns have largely remained numerically similar in the modern era, suggesting that improved surgical techniques may not adequately make up for the high postoperative recurrence rates. In fact, studies show that, of incidental gallbladder cancer findings on non-oncologic cholecystectomy, 25–40% of patients harbor additional disease, largely in regional lymphatics (9,13). An even higher risk of locoregional occurs with margin positive resection, leading some to conclude that this population may particularly benefit from radiation therapy (11). The NCCN lists adjuvant CRT as a possible option in this circumstance as well (2).

Full table
Historically, treatment of patients with localized inoperable disease with definitive chemotherapy plus radiation therapy has been modestly effective (14). In-field local progression 1 to 2 years after treatment has been common because historically definitive doses have been limited to a bioequivalent dose of 50 Gy by the tolerance of the nearby duodenum, complicated by respiratory motion. These limitations of treatment provide a rationale for biologically effective dose (BED)-escalated radiation therapy (ablative radiation, discussed subsequently) made possible by the evolution of organ motion management and stereotactic treatment techniques. Moreover, administering ablative radiation following standard-of-care chemotherapy is probably the only definitive (non-palliative) therapy for inoperable patients. As such, improvements in systemic therapy over the current decade (15), and potentially extrapolating the successes of multi-agent regimens in pancreatic cancer (16,17) (randomized trials (e.g., clinicaltrials.gov NCT03768414 of gemcitabine plus Cisplatin with or without nab-paclitaxel) continue to accrue in biliary cancers), could shift contemporary patterns of failure more towards locoregional recurrences, which may be more conducive towards utilizing RT as a curative-intent modality.
Adjuvant radiotherapy
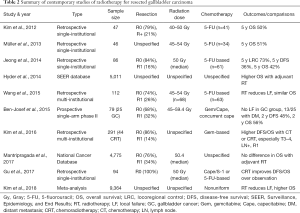
Table 2 summarizes relevant details of contemporary (published over the current decade) studies assessing adjuvant radiation therapy (18-27). Of note, the included studies comprised a majority of R0 resections, most commonly delivered radiation therapy at conventionally fractionated doses between 45–54 Gy, and utilized concurrent 5-fluorouracil (or capecitabine) based chemotherapy. A few noteworthy studies will be further highlighted below.
Full table
Two large national database studies analyzing over 4,500 patients each, were comparative analyses of adjuvant radiation therapy versus lack thereof. The first publication (21) demonstrated an OS benefit with adjuvant radiation therapy following propensity matching; however, the unadjusted cohorts showed that radiation therapy was associated with higher 1-year OS and lower 5-year OS. This implies a high degree of selection bias, possibly explained by the lack of chemotherapy information (or radiation therapy dose) in the database, thus potentially including subjects without chemotherapy (or suboptimal receipt) and/or palliative radiation therapy. The second investigation (25) included information on radiation therapy dose but not chemotherapy agents/timing, and thus was an investigation of post-operative surveillance versus adjuvant chemotherapy plus radiation therapy. No differences in OS were found, even when statistically adjusting for several factors.
A meta-analysis (26) of 9,364 patients from 14 retrospective investigations also showed reduced recurrence and mortality with adjuvant radiation therapy, although chemotherapy details were also discontinuous and non-uniform. Of note, subgroup analyses demonstrated that node-positive and/or incompletely resected patients benefited most from radiation therapy. This is consistent with established data displaying that aggressive management for these high-risk features may be most meaningfully beneficial to outcomes (28,29).
Lastly, the Southwest Oncology Group (SWOG) S0809 study (23), a single-arm phase II trial of adjuvant CRT for resected (pT2–4 or N1 or R1) extrahepatic cholangiocarcinoma (n=54) and GC (n=25), is the only prospective study that has been reported. Protocol therapy consisted of postoperative gemcitabine/capecitabine; if progression did not occur, chemotherapy plus radiation therapy (concurrent capecitabine and intensity-modulated radiation therapy in most cases) was delivered. Radiation therapy consisted of 45 Gy elective nodal irradiation and 54–59.4 Gy to the tumor bed depending on surgical margin status. At median follow-up of 35 months, 2-year OS (primary endpoint) was 65% (67% in R0 cases, 60% in R1 cases) and 2-year disease-free survival was 52%. Corresponding numbers for the gallbladder cancer subjects were 56% and 48%, respectively. The two-year local failure rate was 8% for gallbladder cancer and 11% overall. Of note, 14 subjects developed local failure (none of whom had gallbladder cancer), but most (n=9) had concomitant distant metastasis. Because the 2-year OS was higher than expected based on power calculations, together with the tolerability of the chemotherapy plus radiation therapy regimen, the authors concluded that the trial met its primary endpoint.
Recently, the American Society of Clinical Oncology (ASCO) published guidelines for gallbladder cancer and other biliary cancers, endorsing adjuvant capecitabine following resection and chemotherapy plus radiation therapy for R1 cases (30). Publication of the accruing Perioperative Therapy Preoperative Chemotherapy Versus Chemoradiotherapy in Locally Advanced Gall Bladder Cancers (POLCAGB) trial (NCT02867865), which randomizes patients with gallbladder cancer to perioperative gemcitabine plus cisplatin versus chemotherapy plus radiation therapy (50–55 Gy to gross disease and 45 Gy to subclinical infiltration, concurrent with gemcitabine), could further impact ASCO and NCCN guidelines.
Definitive radiotherapy
Because unresected gallbladder cancer is near-universally fatal, most studies have utilized palliative chemotherapy for this circumstance; however, Table 3 displays contemporary (published over the current decade) investigations evaluating definitive radiation therapy (31-33). These are largely limited to big data studies and/or encompass a minority of gallbladder cancer cases. Both database studies observed a higher OS in patients having received chemotherapy plus radiation therapy over chemotherapy alone; this is notable because neither accounted for RT dose, implying that the effect of RT persisted despite the likely inclusion of palliative radiation therapy. However, owing to inherent limitations of national databases, these findings should be considered hypothesis-generating at the present time.

Full table
There is evidence for dose-response in gallbladder cancer. A study illustrated 5-year local control of 100% for patients having received >54 Gy, as compared to 65% for ≤54 Gy (34). Moreover, the initial site of progression for biliary neoplasms undergoing conventionally-fractionated radiation therapy is local (72%) rather than DM (15%) (6). As a result, with contemporary radiation therapy technology (e.g., high-quality image guidance, adaptive planning, and internal organ motion management), ablative radiation therapy has become an active area of further investigation. Ablation refers to high radiation therapy doses per fraction, thus allowing for much higher BEDs than afforded by conventional (1.8–2 Gy per fraction) fractionation. In addition to notable successes in pancreatic cancer (35), ablative radiation therapy was examined in an investigation of 79 unresected intrahepatic cholangiocarcinomas, most of which underwent chemotherapy prior to radiation therapy (36). This study compared two cohorts, one with BED ≤ 80.5 Gy (most common regimens 50.4 Gy/28 fractions or 58.1 Gy/15 fractions) and the other with BED >80.5 Gy (67.5 Gy/15 fractions or 75 Gy/25 fractions). Radiation therapy dose, when analyzed as a continuous variable in a multivariable model (also accounting for tumor size), independently predicted for higher local control and OS. For subjects having received >80.5 Gy BED, the 3-year local control and OS were impressively high (78% and 73%, respectively); this is noteworthy considering that the median tumor size in all-comers was 8 cm. Ablative radiation therapy was also tolerated well, with few high-grade events and no radiation therapy-induced liver disease.
An emerging modality with which to deliver ablative radiation therapy in a potentially safer manner is proton beam therapy. Proton beam therapy is a highly conformal modality that yields a sharp dose drop-off between the distal edge of the tumor and surrounding normal tissue, owing to the heavy size of the proton and its associated Bragg peak. Although there have been no dedicated series of proton beam therapy for gallbladder cancer, several studies of liver tumors and cholangiocarcinoma (37-39) along with a phase II trial (40) illustrate favorable safety profiles. The role of proton beam therapy for liver and biliary tumors will be better addressed with a number of ongoing randomized trials, including some that employ dose-escalation.
Treatment sequencing
Although management of gallbladder cancer (and other biliary neoplasms) has traditionally involved up-front resection, neoadjuvant therapy an emerging paradigm with a logical rationale in gallbladder cancer. Although there remain concerns regarding tumor progression during neoadjuvant therapy as well as postoperative complications thereafter, neoadjuvant chemotherapy or chemotherapy plus radiation therapy has several advantages. These include downstaging a proportion of initially unresectable cases so as to receive curative resection, reduction of the R+ rate, evaluation of tumor biology, and avoidance of administering radiation therapy in the hypoxic postoperative setting (41,42). Furthermore, preliminary data from the randomized Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1) trial shows that preoperative therapy may offer locoregional, disease-free, and OS advantages as compared to up-front resection (43).
Both chemotherapy alone and chemotherapy plus radiation therapy have been utilized in the literature. Although in the adjuvant setting, chemotherapy alone was administered in the SWOG S0809 trial (23) and was effective to better address micrometastatic disease and select for favorable biology prior to chemotherapy plus radiation therapy. Conversely, up-front chemotherapy plus radiation therapy could avoid the excess toxicities from induction chemotherapy and may better address the risk of local progression than systemic therapy alone.
Most available data for neoadjuvant chemotherapy or chemotherapy plus radiation therapy are retrospective in nature, and are summarized in a recent systematic review (44). From this investigation, 40% of all subjects were able to eventually undergo surgery; for the limited studies of neoadjuvant chemotherapy plus radiation therapy, the rate of post-therapy partial/complete response ranged from 40–70%. These values are encouraging, in part because most radiation therapy approaches have been conventionally fractionated; together with the equally promising data regarding ablative radiation therapy, it is certainly possible that neoadjuvant ablative radiation therapy may offer an even higher ability to proceed with resection. Hence, neoadjuvant approaches represent an exciting frontier that warrants further prospective investigation.
Conclusions
This review describes the contemporary landscape of radiation therapy for gallbladder cancer, chiefly highlighting the rationale to reduce local failure in the adjuvant setting and locoregional disease burden in the definitive setting. Clinical data are limited and of low quality, but nevertheless remain encouraging, especially for high-risk (R+ and/or node-positive) cases in the adjuvant setting and ablative radiation therapy in the definitive setting. Application of advanced radiation therapy technologies such as soft tissue image guidance, adaptive planning, internal organ motion management, and PBT may better facilitate safe delivery of ablative RT. Lastly, neoadjuvant chemotherapy plus radiation therapy is an emerging paradigm that may allow more patients to receive curative resection, which should also be further elucidated going forward.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Dixon E, Vollmer CM Jr, Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg 2005;241:385-94. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Hepatobiliary Cancers. Version 2.2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed June 25, 2019.
- Iwasaki Y, Todoroki T, Fukao K, et al. The role of intraoperative radiation therapy in the treatment of bile duct cancer. World J Surg 1988;12:91-8. [Crossref] [PubMed]
- Miyazaki M, Ito H, Nakagawa K, et al. Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery 1998;123:131-6. [Crossref] [PubMed]
- Nagorney DM, Donohue JH, Farnell MB, et al. Outcomes after curative resections of cholangiocarcinoma. Arch Surg 1993;128:871-7. [Crossref] [PubMed]
- Crane CH, Macdonald KO, Vauthey JN, et al. Limitations of conventional doses of chemoradiation for unresectable biliary cancer. Int J Radiat Oncol Biol Phys 2002;53:969-74. [Crossref] [PubMed]
- Kopelson G, Galdabini J, Warshaw AL, et al. Patterns of failure after curative surgery for extra-hepatic biliary tract carcinoma: Implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 1981;7:413-7. [Crossref] [PubMed]
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of Initial Disease Recurrence after Resection of Gallbladder Carcinoma and Hilar Cholangiocarcinoma: Implications for Adjuvant Therapeutic Strategies. Cancer 2003;98:1689-700. [Crossref] [PubMed]
- Barreto SG, Pawar S, Shah S, et al. Patterns of failure and determinants of outcomes following radical re-resection for incidental gallbladder cancer. World J Surg 2014;38:484-9. [Crossref] [PubMed]
- Margonis GA, Gani F, Buettner S, et al. Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB (Oxford) 2016;18:872-8. [Crossref] [PubMed]
- Kim TG. Patterns of initial failure after resection for gallbladder cancer: implications for adjuvant radiotherapy. Radiat Oncol J 2017;35:359-67. [Crossref] [PubMed]
- Choi HS, Kang KM, Jeong BK, et al. Patterns of failure after resection of extrahepatic bile duct cancer: implications for adjuvant radiotherapy indication and treatment volumes. Radiat Oncol 2018;13:85. [Crossref] [PubMed]
- Shukla PJ. Revision surgery for incidental gallbladder cancer: factors influencing operability and further evidence for T1b tumours. HPB (Oxford) 2008;10:43-7. [Crossref] [PubMed]
- Fareed MM, DeMora L, Esnaola NF, et al. Concurrent chemoradiation for resected gall bladder cancers and cholangiocarcinomas. J Gastrointest Oncol 2018;9:762-8. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Kim K, Chie EK, Jang Y, et al. Postoperative chemoradiotherapy for gallbladder cancer. Strahlenther Onkol 2012;188:388-92. [Crossref] [PubMed]
- Müller B, Sola JA, Carcamo M, et al. Adjuvant chemoradiation for resected gallbladder cancer: Treatment strategies for one of the leading causes of cancer death in Chilean women. Indian J Cancer 2013;50:184-8. [Crossref] [PubMed]
- Jeong Y, Park J, Lee Y, et al. Postoperative Radiotherapy for Gallbladder Cancer. Anticancer Res 2014;34:5621-9. [PubMed]
- Hyder O, Dodson RM, Sachs T, et al. Impact of adjuvant external beam radiotherapy on survival in surgically resected gallbladder adenocarcinoma: A propensity score–matched Surveillance, Epidemiology, and End Results analysis. Surgery 2014;155:85-93. [Crossref] [PubMed]
- Wang J, Narang AK, Sugar EA, et al. Evaluation of Adjuvant Radiation Therapy for Resected Gallbladder Carcinoma: A Multi-institutional Experience. Ann Surg Oncol 2015;22 Suppl 3:S1100-6. [Crossref] [PubMed]
- Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: A phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol 2015;33:2617-22. [Crossref] [PubMed]
- Kim Y, Amini N, Wilson A, et al. Impact of Chemotherapy and External-Beam Radiation Therapy on Outcomes among Patients with Resected Gallbladder Cancer: A Multi-institutional Analysis. Ann Surg Oncol 2016;23:2998-3008. [Crossref] [PubMed]
- Mantripragada KC, Hamid F, Shafqat H, et al. Adjuvant Therapy for Resected Gallbladder Cancer: Analysis of the National Cancer Data Base. J Natl Cancer Inst 2016;109:djw202. [Crossref] [PubMed]
- Kim BH, Kwon J, Chie EK, et al. Adjuvant Chemoradiotherapy is Associated with Improved Survival for Patients with Resected Gallbladder Carcinoma: A Systematic Review and Meta-analysis. Ann Surg Oncol 2018;25:255-64. [Crossref] [PubMed]
- Gu B, Qian L, Yu H, et al. Concurrent Chemoradiotherapy in Curatively Resected Gallbladder Carcinoma: A Propensity Score–Matched Analysis. Int J Radiat Oncol Biol Phys 2018;100:138-45. [Crossref] [PubMed]
- Borghero Y, Crane CH, Szklaruk J, et al. Extrahepatic bile duct adenocarcinoma: patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone. Ann Surg Oncol 2008;15:3147-56. [Crossref] [PubMed]
- Wang SJ, Lemieux A, Kalpathy-Cramer J, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 2011;29:4627-32. [Crossref] [PubMed]
- Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol 2019;37:1015-27. [Crossref] [PubMed]
- Pollom EL, Alagappan M, Park LS, et al. Does radiotherapy still have a role in unresected biliary tract cancer? Cancer Med 2017;6:129-41. [Crossref] [PubMed]
- Verma V, Surkar SM, Brooks ED, et al. Chemoradiotherapy Versus Chemotherapy Alone for Unresected Nonmetastatic Gallbladder Cancer: National Practice Patterns and Outcomes. J Natl Compr Canc Netw 2018;16:59-65. [Crossref] [PubMed]
- Bisello S, Buwenge M, Palloni A, et al. Radiotherapy or Chemoradiation in Unresectable Biliary Cancer: A Retrospective Study. Anticancer Res 2019;39:3095-100. [Crossref] [PubMed]
- Kresl JJ, Schild SE, Henning GT, et al. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys 2002;52:167-75. [Crossref] [PubMed]
- Reyngold M, Parikh P, Crane CH. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol 2019;14:95. [Crossref] [PubMed]
- Tao R, Krishnan S, Bhosale PR, et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J Clin Oncol 2016;34:219-26. [Crossref] [PubMed]
- Makita C, Nakamura T, Takada A, et al. Clinical outcomes and toxicity of proton beam therapy for advanced cholangiocarcinoma. Radiat Oncol 2014;9:26. [Crossref] [PubMed]
- Ohkawa A, Mizumoto M, Ishikawa H, et al. Proton beam therapy for unresectable intrahepatic cholangiocarcinoma. J Gastroenterol Hepatol 2015;30:957-63. [Crossref] [PubMed]
- Verma V, Lin SH, Simone CB 2nd, et al. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review. J Gastrointest Oncol 2016;7:644-64. [Crossref] [PubMed]
- Hong TS, Wo JY, Yeap BY, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol 2016;34:460-8. [Crossref] [PubMed]
- Macdonald OK, Crane CH. Palliative and postoperative radiotherapy in biliary tract cancer. Surg Oncol Clin N Am 2002;11:941-54. [Crossref] [PubMed]
- Verma V, Li J, Lin C. Neoadjuvant Therapy for Pancreatic Cancer: Systematic Review of Postoperative Morbidity, Mortality, and Complications. Am J Clin Oncol 2016;39:302-13. [Crossref] [PubMed]
- Van Tienhoven G, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): A randomized, controlled, multicenter phase III trial. J Clin Oncol 2018;36:LBA4002. [Crossref]
- Hakeem AR, Papoulas M, Menon KV. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - A systematic review. Eur J Surg Oncol 2019;45:83-91. [Crossref] [PubMed]


