The association of KRAS mutation with primary tumor location and survival in patients undergoing resection of colorectal cancers and synchronous liver metastases
Introduction
Colorectal cancer has an annual global incidence of 1.65 million cases (1). At the time of diagnosis, up to 25% of patients already have liver metastases and an additional 25% will develop hepatic metastases during the course of their disease (2). The median overall survival (OS) for patients with metastatic colorectal cancer that is treated with a palliative intent with multi-agent chemotherapy is approximately 30 months (3). Although surgical indications and timing of resections in colorectal cancer and liver metastases are still a matter of debate, hepatic metastasectomy with resection of the primary tumor remains the only potentially curative treatment (3-5). Mutations of the Kirsten Rat Sarcoma viral antigen homolog (KRAS) gene are found in about 40% of patients (6) with metastatic colorectal cancer. The determination of KRAS status is a relevant prognostic characteristic in these patients, as mutated KRAS (m-KRAS) reflects a poor response to anti-EGFR immunotherapy (3) and is associated with an increased cumulative incidence of metastatic disease as well as an adverse prognosis (6-8).
In recent years, the molecular pathways of oncogenesis in colorectal cancer and the intrinsic differences between right-sided and left-sided lesions have been investigated. Two meta-analyses (6,9) of institutional studies evaluating the prognostic significance of m-KRAS in patients treated with surgical resection of primary colorectal cancer and synchronous hepatic metastases, observed that m-KRAS was associated with decreased OS and disease-free survival (DFS). More recently, a study (10) using the National Cancer Database (NCDB) confirmed the negative prognostic impact of m-KRAS in colorectal cancer with synchronous liver metastases and demonstrated that m-KRAS was associated with right-sided lesions and African-American ethnicity. Two main limitations of the NCDB are the lack of data on disease-specific survival (DSS) and the limited generalizability to the United States (U.S.) population as this database only collects data from the Commission on Cancer (CoC) affiliated Hospitals.
Therefore, aim of this study was to analyze the impact of m-KRAS on DSS as well as its associated risk factors in patients undergoing resection of colorectal cancer and synchronous liver metastases in a U.S. population-based cohort utilizing the Surveillance, Epidemiology and End Results (SEER) database.
Methods
The SEER Program (11) provides information on cancer statistics within the U.S. It is supported by the Surveillance Research Program (SRP) within the National Cancer Institute (NCI). Data were available for a total of 18 registries representing approximately 30% of the U.S. population. The population covered by SEER is representative of the general U.S. population with regards to measures of income and education level. However, the SEER population presents a higher proportion of foreign-born patients as compared to the general U.S. population. SEER employs the International Classification of Diseases for Oncology, third edition (ICD-O-3) for histology classification (12).
In order to select patients diagnosed with colorectal cancer and synchronous liver metastases between 2010 and 2015, the Incidence—SEER 18 Regs Custom Data Colon and Rectum Database was queried (13). The selection of patients was conducted as follows: age older than 18 years, Stage IV colorectal adenocarcinoma with isolated liver metastases, resection of the primary tumor, nonprimary surgical procedure to distant site (i.e., patients undergoing liver resection) and known mutational status of KRAS.
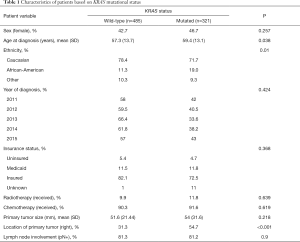
Demographic variables included in the analyses were gender, age at diagnosis, year of diagnosis, ethnicity and insurance status. Clinicopathologic variables included administration of chemotherapy and radiation therapy, surgical treatment of the primary tumor and metastatic lesions, location and size of the primary tumor, involvement of the lymph nodes and KRAS status of the primary colorectal cancer. Primary tumor location was classified as ‘right-sided’ for lesions located from the cecum to the transverse colon and as ‘left-sided’ for lesions located from the splenic flexure to the rectum.
Statistical analyses
Descriptive statistics of demographic and clinicopathologic variables were performed. Student’s t-test and Chi-square test were performed to compare continuous and discrete variables, respectively. Univariate and multivariate binary logistic regression was used to recognize factors associated with m-KRAS and odds ratios (OR) and 95% CI were calculated. DSS was estimated with the Kaplan-Meier method and the log-rank test was used to evaluate statistical significance of the differences in survival. Cox proportional hazard regression was used for the multivariable survival model. Hazard ratios (HR) and 95% confidence intervals were computed for the power of association between each variable and survival.
Data analyses were performed using SEER*Stat software (14) and Statistical Package for the Social Sciences (SPSS) software (version 20.0; SPSS Inc., Chicago, IL, USA). P values were considered significant if <0.05 and all tests were two-sided. Considering that data included in the SEER Database is publicly available and de-identified, approval by an institutional review board was not considered necessary for the current study.
Results
Patient characteristics
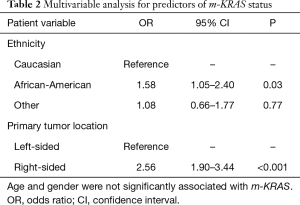
A total number of 806 patients with colorectal cancer and synchronous liver metastases and documented KRAS mutational status who underwent surgical treatment of their primary tumor and liver metastases were retrieved in the SEER Database. m-KRAS was present in 321 cases (39.8%). Overall m-KRAS was associated with older age (59.4 vs. 57.3 years, P=0.038; Table 1) and African-American ethnicity (19.0% vs. 11.3%, P=0.01), but was not related to gender, insurance status or year of diagnosis. Furthermore, m-KRAS was more commonly found in right-sided primary lesions, when compared to left-sided lesions (54.7% vs. 31.3%, P<0.001). Clinicopathologic variables, such as primary tumor size, lymph node involvement and radio-chemotherapy administration were not significantly associated with m-KRAS. On multivariable analysis, right-sided lesions (OR 2.56, 95% CI: 1.90–3.44, P<0001) and African-American ethnicity (OR 1.58, 95% CI: 1.05–2.40, P=0.03) were significantly associated with m-KRAS status (Table 2).
Full table
Full table
Survival analyses
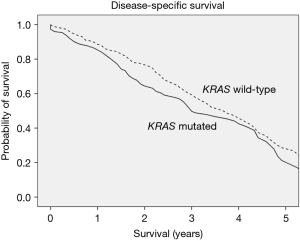
Compared to wt-KRAS, m-KRAS status was associated with decreased DSS; 3- and 5-year DSS were 59% vs. 50%, and 29% vs. 21%, respectively (P=0.024; Figure 1). Similarly, patients with right-sided lesions demonstrated an increase in disease-specific mortality, with a 5-year DSS of 19%, as compared to 29% for left-sided lesions (P<0.001).
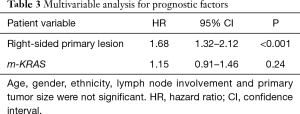
After adjusting for the available confounders, patients with right-sided lesions had a decreased DSS (HR 1.68, 95% CI: 1.32–2.12, P<0.001; Table 3). Furthermore, m-KRAS demonstrated a trend toward decreased DSS (HR 1.15, 95% CI: 0.91–1.46, P=0.24).
Full table
Discussion
The present study investigated the associations between KRAS mutational status, primary tumor location and survival in a cohort of 806 patients from a population-based dataset. African-American ethnicity and right-sided lesions were independently associated with m-KRAS. Moreover, m-KRAS and the presence of a right-sided primary lesion were negative prognostic factors in patients with colorectal cancer and synchronous liver metastases undergoing surgical resection of both their primary colorectal cancer and liver metastases.
Given its relatively high incidence, the relative ease of detectability, and the various targeted therapeutic options available (3,4), m-KRAS is an important prognostic consideration in the management of metastatic colorectal cancer. In the context of multi-disciplinary strategies, the decision-making process is often driven by clinical and radiological features, rather than pathologic and biologic elements. However, the mutational status of KRAS may predict the responsiveness to upfront chemotherapy in presence colorectal cancer and synchronous liver metastases (15) and may also explain intrinsic aggressive tumor biology (16,17), resulting in increased risk of progression and relapse after resection. m-KRAS is acquired early in the mutational cascade, it remains stable over the course of the disease, and has a high concordance between primary tumor and liver metastases (18). Several preoperative clinical risk scores for patients presenting with resectable colorectal cancer and synchronous liver metastases were developed (19-22) based on institutional cohorts, although there have been concerns regarding the absence of external validation of these scores as well as the accuracy and applicability of the reported prognostic factors (23). Therefore, using current knowledge of the mutational profile of colorectal cancer to guide clinical practice could harbor the potential of improving oncologic outcomes. This has led to the creation of the Genetic and Morphological Evaluation (GAME) Score (24), which is based on morphologic and biologic tumor information and represents the first clinical risk score including the genetic status (KRAS) of the primary tumor. The score was developed using a derivation cohort, including 502 patients, and an external validation cohort (747 patients) and its discriminatory capacity resulted to be superior to other institutionally derived scores. Integrating genetic, biologic and clinical information not only is likely to improve comparison of different cohorts, but may also guide treatment selection and provide relevant prognostic details (24).
The proportion of patients with m-KRAS in the current study (39.8%) was consistent with previous reports in the literature (14–46%). In an analysis of all Stage IV colorectal cancers within the SEER database, Charlton et al. (25) reported an overall mutation rate of 44% among 6794 patients. Similarly, in their study utilizing the NCDB, Goffredo et al. (10) reported m-KRAS in 42% out of 2,655 patients who underwent surgical treatment of both their primary colorectal cancer and isolated liver metastases. Two meta-analyses of institutional studies by Brudvik et al., that included 14 studies with 1,809 cases, and Tosi et al., which included 11 studies with 1,369 patients, reported an overall m-KRAS incidence of 30.6% and 34.3%, respectively. While there was substantial overlap between these two studies, they differed in that Tosi et al. used a more stringent criteria for inclusion in their analysis and added three newer studies (6,9). Both these meta-analyses demonstrated that m-KRAS was negatively associated with OS and relapse free survival, irrespective of chemotherapy agent received.
In our cohort, the proportion of patients with a right-sided cancer within the m-KRAS subgroup was high at 54%, as compared to 31% within the wt-KRAS subgroup. In a meta-analysis including 66 studies and 1,437,846 patients, Petrelli et al. (26) reported that right-sided lesions were associated with worse OS. There have been several explanations in the literature for this difference in survival based on location. Firstly, surgical approaches to right-sided and left- sided colon/rectal cancer are different: while the standard of care is well defined for left-sided and rectal lesions, the optimal surgical resection for right-sided lesions remains debated, particularly in regards to the extent of mesocolic excision and lymphadenectomy (27,28). Secondly, benefits from anti-EGFR agents is less pronounced in right-sided lesions, as reported by two recent trials (29,30). Finally, right-sided colon cancer is associated with increased incidence of genetic mutations [microsatellite instability (MSI), BRAF and KRAS mutations] which may explain the survival difference in this subgroup of patients (31).
In the current study, right-sided tumors and African-American ethnicity were significantly associated with the presence of m-KRAS status. Right-sided lesions, but not African-American ethnicity, were associated with lower DSS on multivariable survival analysis. Using the NCDB, Goffredo et al. found comparable results in a U.S. based national cohort of patients (10), even though the NCDB and SEER databases are different in terms of quality and origin of data. The former collects data from Commission on Cancer-accredited cancer program registries, capturing around 70% of all newly diagnosed cases of cancer at an institutional level; the latter gathers data from population-based cancer registries and covers 34.6% of the U.S. population. Notwithstanding the possible overlapping between the two datasets, the reproducibility of results between these two studies underscores the validity of the association between m-KRAS and survival outcomes. Our findings appear to support the hypothesis that specific tumor biology and mutational status may be related to primary tumor location and could be the main driver for worse outcomes.
The present study has several limitations. Firstly, the retrospective nature of the analysis of the SEER database might be flawed by intrinsic biases of large databases. Secondly, the SEER is a population-based dataset and several potentially significant variables are not collected routinely. Thirdly, the vast range of therapeutic possibilities, including type of surgical resection, various lines of chemotherapy, radiotherapy and their temporal combination, could not be investigated in depth. Finally, the accuracy and extent of surgical treatment as well as other therapeutic maneuvers could not be evaluated.
Conclusions
In conclusion, the present study suggests a negative prognostic value of m-KRAS and right-sided lesions in a population-based cohort of patients undergoing resection of colorectal cancer and synchronous liver metastases. Our data show that African-American ethnicity and right-sided lesions were associated with m-KRAS. The mutational status of KRAS is likely to be associated with unfavorable tumor biology leading to decreased survival and should be discussed in the context of multi-disciplinary management of patients with colorectal cancer and synchronous liver metastases.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The data included in the SEER Database is publicly available and de-identified, approval by an institutional review board was not considered necessary for the current study.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- van der Pool AE, Damhuis RA, Ijzermans JN, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis 2012;14:56-61. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Adam R, Aimery de Gramont A, Joan Figueras AG, et al. The Oncosurgery Approach to Managing Liver Metastases from Colorectal Cancer: A Multidisciplinary International Consensus. Oncologist 2012;17:1225-39. [Crossref] [PubMed]
- Chow FCL, Chok KSH. Colorectal liver metastases: An update on multidisciplinary approach. World J Hepatol 2019;11:150-72. [Crossref] [PubMed]
- Tosi F, Magni E, Amatu A, et al. Effect of KRAS and BRAF Mutations on Survival of Metastatic Colorectal Cancer After Liver Resection: A Systematic Review and Meta-Analysis. Clin Colorectal Cancer 2017;16:e153-63. [Crossref] [PubMed]
- Kim MJ, Lee HS, Kim JH, et al. Different metastatic pattern according to the KRAS mutational status and site-specific discordance of KRAS status in patients with colorectal cancer. BMC Cancer 2012;12:347. [Crossref] [PubMed]
- Cejas P, López-Gómez M, Aguayo C, et al. KRAS mutations in primary colorectal cancer tumors and related metastases: A potential role in prediction of lung metastasis. PLoS One 2009;4:e8199. [Crossref] [PubMed]
- Brudvik KW, Kopetz SE, Li L, et al. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg 2015;102:1175-83. [Crossref] [PubMed]
- Goffredo P, Utria AF, Beck AC, et al. The Prognostic Impact of KRAS Mutation in Patients Having Curative Resection of Synchronous Colorectal Liver Metastases. J Gastrointest Surg 2019;23:1957-63. [PubMed]
- Available online: https://seer.cancer.gov/
- Fritz A, Jack A, Shanmugarathnam K, et al. International Classification of Disease for Oncology. 3rd ed. Geneva: World Health Organization, 2000.
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Custom Data Colon and Rectum (with SSF 9 - in-house and treatment fields. Available online: www.seer.cancer.gov
- Surveillance Research Program, National Cancer Institute SEER*Stat software (version 8.3.5). Available online: www.seer.cancer.gov/seerstat
- Zimmitti G, Shindoh J, Mise Y, et al. RAS Mutations Predict Radiologic and Pathologic Response in Patients Treated with Chemotherapy Before Resection of Colorectal Liver Metastases. Ann Surg Oncol 2015;22:834-42. [Crossref] [PubMed]
- Italiano A, Hostein I, Soubeyran I, et al. KRAS and BRAF Mutational Status in Primary Colorectal Tumors and Related Metastatic Sites: Biological and Clinical Implications. Ann Surg Oncol 2010;17:1429-34. [Crossref] [PubMed]
- Margonis GA, Kim Y, Spolverato G, et al. Association between specific mutations in KRAS codon 12 and colorectal liver metastasis. JAMA Surg 2015;150:722-9. [Crossref] [PubMed]
- Knijn N, Mekenkamp LJM, Klomp M, et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer 2011;104:1020-6. [Crossref] [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical Score for Predicting Recurrence After Hepatic Resection for Metastatic Colorectal Cancer. Ann Surg 1999;230:309. [Crossref] [PubMed]
- Iwatsuki S, Dvorchik I, Madariaga JR, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg 1999;189:291-9. [Crossref] [PubMed]
- Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254-62. [Crossref] [PubMed]
- Rees M, Tekkis PP, Welsh FKS, et al. Evaluation of Long-term Survival After Hepatic Resection for Metastatic Colorectal Cancer. Ann Surg 2008;247:125-35. [Crossref] [PubMed]
- Zakaria S, Donohue JH, Que FG, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg 2007;246:183-91. [Crossref] [PubMed]
- Margonis GA, Sasaki K, Gholami S, et al. Genetic And Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg 2018;105:1210-20. [Crossref] [PubMed]
- Charlton ME, Kahl AR, Greenbaum AA, et al. KRAS Testing, Tumor Location, and Survival in Patients With Stage IV Colorectal Cancer: SEER 2010–2013. J Natl Compr Canc Netw 2017;15:1484-93. [Crossref] [PubMed]
- Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic survival associated with left-sided vs right-sided colon cancer a systematic review and meta-analysis. JAMA Oncol 2017;3:211-9. [Crossref] [PubMed]
- Vogel JD, Eskicioglu C, Weiser MR, et al. The American society of colon and rectal surgeons clinical practice guidelines for the treatment of colon cancer. Dis Colon Rectum 2017;60:999-1017. [Crossref] [PubMed]
- Bertelsen CA, Neuenschwander AU, Jansen JE, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 2015;16:161-8. [Crossref] [PubMed]
- Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1o) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016;34:3504. [Crossref]
- Heinemann V, Modest DP, Fischer von Weikersthal L, et al. Gender and tumor location as predictors for efficacy: Influence on endpoints in first-line treatment with FOLFIRI in combination with cetuximab or bevacizumab in the AIO KRK 0306 (FIRE3) trial. J Clin Oncol 2014;32:3600. [Crossref] [PubMed]
- Chiu JW, Krzyzanowska MK, Serra S, et al. Molecular Profiling of Patients With Advanced Colorectal Cancer: Princess Margaret Cancer Centre Experience. Clin Colorectal Cancer 2018;17:73-9. [Crossref] [PubMed]