
The state of hepatic artery infusion chemotherapy in the management of metastatic colorectal cancer to the liver
Rationale for hepatic artery infusion (HAI) chemotherapy
There are approximately 150,000 new cases of colorectal carcinoma diagnosed annually in the United States (1). Approximately 25% of patients present with metastatic disease at the time of diagnosis (2) and over 50% will develop metastasis to the liver at some point in their lifetime (3). Five-year overall survival (OS) in metastatic colorectal cancer (CRC) confined to the liver is approximately 20%, although complete resection can increase 5-year survival to over 50% in selected series (4-7).
Liver resection provides the only chance for cure in patients with colorectal liver metastases (CRLM), however, only 15–20% of patients with CRC metastases confined to the liver are deemed resection candidates at presentation. Most patients with CRC die from metastatic disease, and two-thirds of CRC deaths are due to liver metastases (8).
For patients with initially unresectable CRLM, regional treatment of metastatic disease has been a topic of considerable interest given the underwhelming response rates to systemic chemotherapy alone with median survival of roughly 20 months (9). First line systemic chemotherapy for metastatic CRC includes a fluoropyrimidine combined with other agents in various schedules (10). Despite the advances of modern combination regimens [e.g., 5-FU/leucovorin/oxaliplatin (FOLFOX), 5-FU/leucovorin/irinotecan (FOLFIRI) and 5-FU/leucovorin/oxaliplatin/irinotecan (FOLFOXIRI) and targeted therapies including anti-epidermal growth factor receptor (EGFR) for RAS wild-type tumors, and anti-vascular endothelial growth factor (VEGF) agents], response rates to first line therapy for metastatic CRC range from 34–66%; and up to 30–40% for 2nd line (11). Progression-free survival for first line agents is 5.1–13 months, and decreases to 1.7–7.3 months for second line agents (12). Therefore, HAI chemotherapy has become an attractive strategy for locoregional disease control and possible conversion to resection for patients with initially unresectable liver-dominant disease. HAI chemotherapy takes advantage of the liver anatomy and regional arterial supply to metastatic CRC lesions to deliver high concentration of chemotherapy with minimal systemic side effects. While most of the liver parenchyma derives its blood supply from the portal circulation, metastatic tumors derive their blood supply from the hepatic arteries via angiogenesis as the malignancy grows. Furthermore, the hepatic extraction of chemotherapy agents is much higher when infused through the hepatic artery. For example, one commonly used agent for HAI therapy, floxuridine (FUDR), has 400 times greater concentration in liver tumors when infused through the hepatic artery compared to systemic infusion (13).
HAI therapy has evolved in the last three decades with improved surgical techniques and discovery of new systemic agents. It is now an acceptable first line option in the United States for unresectable CRLM and a treatment option in the adjuvant setting; however, it remains infrequently used. Reasons for this include lack of widespread expertise with HAI therapy, requirement of a multidisciplinary team to manage therapy, and more widespread use of systemic chemotherapy in first-line settings (14).
Methods of implantation
The current gold standard for implantation is laparotomy, with insertion of a catheter into the gastroduodenal artery. Prior to surgery, computed tomography angiogram is used to visualize the hepatic arterial anatomy to assess for aberrant anatomy and caliber of the gastroduodenal artery. Less invasive strategies of implantation, including laparoscopic and robotic placement, have been studied, but so far, none have demonstrated superiority in pump-related complications (15). Percutaneous trans-axillary insertion is associated with decreased length of hospitalization but with significantly higher rate of catheter-related complications (16,17).
Agents used for HAI chemotherapy
5-FU was the first agent to be used for HAI due to its early effectiveness as systemic therapy. Later FUDR, a prodrug of 5-FU, was found to have much higher concentration within tumor, with fewer systemic side effects compared to 5-FU (18). Modern chemotherapeutic agents such as irinotecan and oxaliplatin have been used mostly in Europe and Asia as shown in Table 1 (19-22). These agents have a lower first-pass hepatic extraction compared to FUDR and a different systemic toxicity profile.

Full table
Role of HAI chemotherapy in current guidelines
Current National Comprehensive Cancer Network (NCCN) guidelines for metastatic CRC recommend that if metastatic disease is limited to the liver and is amenable to resection, surgery should be pursued as first line therapy. Adjuvant HAI with systemic therapy can follow, or adjuvant systemic chemotherapy alone. For patients with initially unresectable, liver-dominant disease, HAI with systemic chemotherapy can be pursued or systemic therapy alone. There is increasing popularity to consider earlier initiation of HAI in patients with initially unresectable disease who are chemotherapy-naive to increase resection rates (23). There is currently no role for HAI therapy in the neoadjuvant setting for patients with resectable disease. European Society for Medical Oncology (ESMO) guidelines do not currently recommend HAI (10).
Outcomes of HAI chemotherapy for unresectable disease
Conversion to resection is the goal of HAI and systemic chemotherapy, as resection is independently associated with prolonged OS (6,7). However, resectability of liver metastasis is surgeon and institution dependent, and therefore, reports of conversion to resection rates in available studies are difficult to interpret.
Available treatment options for unresectable CRLM limited to the liver were initially limited to systemic chemotherapy, with low conversion rates and poor OS. The first systemic chemotherapy used for metastatic CRC was systemic 5-FU, with a response rate of only about 20%, and average survival of approximately 11 months (24). Irinotecan and oxaliplatin were later developed and had a higher response rate (30–45%) and a slightly longer median survival of 15–19.5 months (25). In a single institution study from 1988 to 1999, the conversion rate with chemotherapy (which consisted mainly of 5-FU and leucovorin combined with oxaliplatin or irinotecan) was 12.5% (26). In more recent studies using modern combination therapy and targeted therapy, response rates of up to 81% and conversion rates up to 60% are reported with combination FOLFOXIRI (5-FU/oxaliplatin/irinotecan) and bevacizumab (27,28). Modern systemic chemotherapy has increased survival in metastatic CRC patients to 18–30 months; however, disease progression and eventual death secondary to liver failure remain the biggest clinical challenges. Thus, there is a continued interest in HAI and other locoregional therapies including radiofrequency ablation, stereotactic body radiation, and chemoembolization as methods to stave off hepatic disease progression (29).
HAI therapy vs. systemic chemotherapy for unresectable disease
HAI alone was initially compared to available systemic chemotherapies for first-line use for unresectable CRLM. Multiple prospective clinical trials published in the late 1980s to early 1990s comparing HAI with systemic chemotherapy demonstrated superior response rates of HAI therapy but did not show consistent improvements in OS (30-33) (Table 2). To add to the skepticism towards HAI therapy, a 2006 meta-analysis of randomized controlled trials (RCTs) comparing HAI and systemic chemotherapy in unresectable disease showed that there was no survival advantage to HAI alone (35). This analysis, however, had several limitations including, small number of patients from single institutions, old HAI chemotherapy, and allowance for cross over to HAI in patients who had initially failed systemic chemotherapy. To overcome these limitations, a multi-institutional prospective randomized clinical trial, Cancer And Leukemia Group B (CALGB) 9481, investigated response rate in patients receiving HAI FUDR compared to systemic 5-FU only, demonstrating significantly improved survival (24.4 vs. 20 months; P=0.0034) (34). In that study, 40–70% of patients with unresectable hepatic liver metastases who underwent HAI therapy later developed extrahepatic disease; thus, systemic chemotherapy combined with HAI was considered as a practical approach to control both intrahepatic and extrahepatic metastases (36).
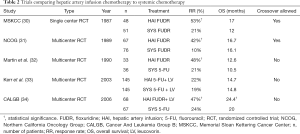
Full table
HAI therapy with systemic chemotherapy was first studied by Safi et al. in 1989 in a phase I prospective study comparing HAI FUDR with HAI FUDR and systemic FUDR. The results of this study showed that addition of systemic FUDR to HAI FUDR therapy was well tolerated, with no significant difference in rates of toxicities between the two groups. However, no significant difference was found in response rate or extrahepatic recurrence (37). Further studies comparing systemic therapy alone with HAI therapy and systemic therapy were done. Many early studies using systemic 5-FU and FUDR showed improved response rates but no significant difference in survival. For example, in 2000, a prospective RCT by Allen-Mersh et al. compared HAI FUDR plus systemic 5-FU and leucovorin against systemic 5-FU and leucovorin alone and found greater response 45% vs. 23%, but no significant difference in survival rates (38).
In 2009, a study of 153 patients randomized to receive HAI FUDR alone or HAI FUDR and systemic 5-FU as first line therapy, demonstrated no difference in response (52.7% vs. 50.6%) and OS (18.0 vs. 19.1 months). Of the variables considered as predictors of tumor response (including age, sex, stage, site of primary tumor, adjuvant therapy after primary tumor resection, extent of liver involvement) response to therapy and lower tumor burden (<50% of liver parenchymal involvement) were the only predictors of OS. OS in patients with <50% liver involvement compared to >50% was significantly greater (21.3 vs. 13.2 months) (39). These results suggest that HAI therapy can be more beneficial if likely responders can be identified through biomarkers such as gene mutational status.
With the introduction of oxaliplatin and irinotecan for systemic therapy in the late 1990s, a series of clinical trials tested the efficacy and safety of HAI with combinations of these modern agents (23) (Table 1). A single arm phase I study of 49 patients by Kemeny et al. at Memorial Sloan Kettering Cancer Center (MSKCC) compared HAI FUDR/dexamethasone added to systemic oxaliplatin and irinotecan in patients with adverse prognostic characteristics (at least 5 hepatic lesions to be enrolled, and 53% pre-treated patients with systemic chemotherapy). Their outcomes showed high response (92%) and conversion to resection rates (47%). Thirty-nine percent underwent R0 resection. Postoperative complications of hematoma and fluid collection occurred in only 2 (9%) patients, and significant toxicities included diarrhea (14%), neutropenia (23%), and neurotoxicity (23%) (40). Similarly, Goere et al. in 2010, analyzed 87 patients who received HAI oxaliplatin with systemic 5-FU and leucovorin, as second line therapy, and 21 of 87 patients (24%) underwent resection, with 5-year OS of 56% (41). More recently, studies continue to show high response rates up to 76% and conversion to resection up to 52% using various combinations of modern agents (42-45) (Table 3).

Full table
Outcomes of adjuvant HAI therapy for resectable disease
Despite achieving R0 resection, relapse rates are over 50% within two years after resection, with 70% of recurrences involving the liver (36,46). The role of adjuvant therapy is thought to target micrometastases within the liver after curative resection of liver metastases. Early HAI studies demonstrated mixed results. While three studies at MSKCC, Greece, and an intergroup study showed increase in recurrence-free survival and OS, a study in Germany by Lorenz et al. did not show any survival benefit (Table 4). The study by Lorenz et al. compared adjuvant HAI of 5-FU to no treatment and showed lower median survival in the HAI group than in the control, and was terminated early (48). In 2004, a Cochrane review of seven available randomized adjuvant HAI studies showed no increase in OS; consequently, adjuvant HAI was not recommended by the authors (51).
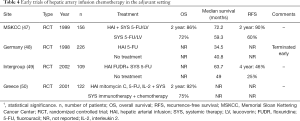
Full table
More recently, a 2016 study by Kemeny et al. followed 287 patients that underwent adjuvant HAI with FUDR and systemic therapy after resection of liver metastases, from 1991 onward. The patients were divided into two groups: those treated before 2003 and after. Results of this study showed greater 5- and 10-year OS in those treated after 2003 compared to before 78% vs. 56%, and 61% vs. 40%, respectively. The rationale for choosing year 2003 as the division point, was that it was in 2003 that RAS mutation status was available at the institution, and 37% of RAS wild-type patients received cetuximab or panitumumab after recurrence. Also, in 2004 institutional protocols shifted to modern combination systemic therapy including FOLFIRI or FOLFOX with bevacizumab. This greater OS in patients treated after 2003 was thought to be secondary to new targeted therapies including cetuximab, panitumumab, and bevacizumab, more aggressive surgical treatment of recurrences, and better imaging (52).
To further delineate the contribution of modern systemic chemotherapy from that of HAI therapy in improving OS, subsequent studies considered whether or not HAI therapy was given with perioperative modern systemic therapy as a subgroup analysis (53). One such study was a 21-year analysis of perioperative HAI therapy that included 2,368 consecutive patients who underwent curative-intent liver resection for CRLM at MSKCC from 1992 to 2012. The results showed prolonged 5-year OS for patients receiving HAI therapy, compared to those treated without HAI (52.9% vs. 37.9%, P<0.001), and also greater 10-year OS (38.0% vs. 23.8%, P<0.001). Subgroup analysis demonstrated that regardless of receiving modern systemic chemotherapy or not, or whether HAI was received in the preoperative or adjuvant setting, there remained a significantly greater OS in the HAI arm (54). For those that received preoperative modern systemic chemotherapy, median OS rates in the HAI arm and the no HAI arm were 77 and 45 months, respectively. For those that did not receive preoperative modern systemic chemotherapy, median OS rates in the HAI arm and the no HAI arm were 55 and 43 months, respectively. These results matched, on a larger scale those of prior studies (55). Results of this study suggest that despite the increased effectiveness of modern systemic chemotherapy, there is still a benefit in OS with the addition of HAI therapy to systemic therapy in the perioperative setting.
Two RCTs are currently underway to further evaluate adjuvant HAI after resection of CRLM. The PUMP trial is a phase III trial underway in the Netherlands that seeks to assess efficacy of adjuvant HAI FUDR therapy in low risk patients. Low risk is defined as no more than 2 of 5 of the following factors: disease-free interval less than 12 months, node-positive CRC, more than 1 CRLM, largest liver metastasis more than 5 cm in diameter, serum carcinoembryonic antigen (CEA) above 200 µg/L (56). Patients are randomized to either resection without any adjuvant therapy (standard of care in the Netherlands) or implantation of pump at time of resection with 6 cycles of HAI FUDR. The primary endpoint of the study is progression-free survival. Secondary endpoints are OS, hepatic progression-free survival, safety, quality of life, and cost effectiveness. The study seeks to validate prior results at MSKCC for adjuvant use of HAI FUDR therapy (47).
PACHA-01 is an ongoing phase II/III trial comparing adjuvant systemic FOLFOX and HAI oxaliplatin + systemic 5-FU in patients with high risk of recurrence, defined as having 4 or more resected CRLM in patients who have undergone R0 or R1 resection and/or thermal ablation (57). The primary objectives are (I) to assess the 18-month hepatic recurrence-free survival in patients treated with HAI oxaliplatin + systemic 5-FU after curative intent surgery, and (II) demonstrate superiority in recurrence-free survival of HAI oxaliplatin compared to systemic oxaliplatin + 5-FU (FOLFOX). Secondary objectives include assessing feasibility, toxicity, and efficacy.
Molecular markers and outcomes of liver resection and HAI therapy
Testing for molecular markers is being increasingly used to guide selection of therapy, predict response to targeted therapies, and to offer more exact prognosis. Current NCCN guidelines recommend all patients with CRC have tissue genotyped for RAS and BRAF mutations, and mismatch repair (MMR) DNA testing or microsatellite instability (MSI) immunohistochemistry. About 20–40% of CRC patients carry a mutation in the RAS gene and about 10% carry a BRAF mutation (58-60). MSI due to defective MMR is present in about 15% of all CRCs (61).
RAS and BRAF mutations have been known to independently predict poor prognosis in metastatic CRC and are both associated with resistance to EGFR targeted therapies. Since the discovery and usage of anti-EGFR antibodies, cetuximab and panitumumab approved in 2004 and 2007 respectively, RAS testing was the earliest molecular marker to became part of NCCN and American Society of Clinical Oncology (ASCO) guidelines in 2009 (62). There is stronger evidence supporting the negative prognostic significance of RAS than BRAF, due to the higher prevalence of RAS in CRC (63). A 2011 study showed increased rate of peritoneal and distant lymph node metastases with BRAF-mutant tumors, and lower OS (10.4 vs. 34.7 months) (59). Another large study at MSKCC showed that BRAF-mutant CRC less commonly presents with disease limited to the liver, and also showed shorter OS, 20 vs. 47 months (P<0.001) when compared to BRAF wild-type (64).
There are few studies on the effect of molecular markers on the outcomes in HAI therapy. In a study of 169 patients with resected CRLM who underwent adjuvant HAI therapy, 3-year recurrence-free survival for KRAS wild-type and KRAS-mutant was 46% and 30%, respectively (P=0.005) (65). KRAS-mutant tumors are also associated with higher cumulative incidence of bone (13.4% vs. 2%), brain (14.5% vs. 2%), and lung metastases (58% vs. 33.2%).
The only large study investigating the effects on RAS status on adjuvant HAI therapy included 674 patients with CRLM who had KRAS testing data at MSKCC. These patients underwent curative-intent hepatic resection, and received either adjuvant HAI with FUDR and systemic chemotherapy or systemic chemotherapy alone. When comparing baseline characteristics, patients in the HAI group had more advanced disease characterized by higher rates of synchronous disease, larger number of tumors, higher clinical risk scores, and rates of 2-stage hepatectomy. Results of the study showed that 5-year OS with HAI was prolonged, regardless of KRAS status. Adjuvant HAI was associated with improved 5-year OS in KRAS wild-type (76% vs. 57%, P<0.001) and in KRAS-mutant (59% vs. 40%, P<0.001) (66). HAI was an independent predictor of OS independent of KRAS status. The results of this study suggest that with the combination of HAI therapy and systemic therapy, KRAS-mutant tumors can achieve a 5-year OS that rivals that of KRAS wild-type tumors treated with systemic chemotherapy only (59% vs. 57%) (66).
Conclusions
HAI chemotherapy has come a long way through improved surgical technique, increased institutional experience with pump implantation and monitoring, and discovery of new therapeutic agents. The future of HAI chemotherapy is hopeful as trials testing combination of chemotherapies are underway, and the optimal regimen and sequence of therapy are being determined. Furthermore, promising results of converting initially unresectable patients to resection may mean possible translation into use as neoadjuvant therapy. Identification of those patients likely to benefit from HAI through the identification of molecular markers continues to be of interest especially as new targeted systemic therapies become available. As the intricacies of the molecular pathways in CRC tumorigenesis are identified, an increasingly more targeted approach to CRC treatment will be sought. More clinical trials of HAI therapy distinguishing the response by CRC of different genotypic mutational status are warranted.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Van Cutsem E, Oliveira J. ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20 Suppl 4:61-3. [PubMed]
- Welch JP, Donaldson GA. The clinical correlation of an autopsy study of recurrent colorectal cancer. Ann Surg 1979;189:496-502. [PubMed]
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575-80. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [Crossref] [PubMed]
- Wei AC, Greig PD, Grant D, et al. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 2006;13:668-76. [Crossref] [PubMed]
- Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 2006;94:982-99. [Crossref] [PubMed]
- Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol 1986;150:195-203. [Crossref] [PubMed]
- Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol 2008;26:5721-7. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii1-9. Erratum in: The ESMO Guidelines Committee would like to publish the following corrections to manuscripts published in 2014. [Ann Oncol 2015]. [Crossref] [PubMed]
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. [Crossref] [PubMed]
- Holch J, Stintzing S, Heinemann V. Treatment of Metastatic Colorectal Cancer: Standard of Care and Future Perspectives. Visc Med 2016;32:178-83. [Crossref] [PubMed]
- Dizon DS, Schwartz J, Kemeny N. Regional chemotherapy: a focus on hepatic artery infusion for colorectal cancer liver metastases. Surg Oncol Clin N Am 2008;17:759-71. viii. [Crossref] [PubMed]
- Karanicolas PJ, Metrakos P, Chan K, et al. Hepatic arterial infusion pump chemotherapy in the management of colorectal liver metastases: expert consensus statement. Curr Oncol 2014;21:e129-36. [Crossref] [PubMed]
- Qadan M, D'Angelica MI, Kemeny NE, et al. Robotic hepatic arterial infusion pump placement. HPB (Oxford) 2017;19:429-35. [Crossref] [PubMed]
- Arru M, Aldrighetti L, Gremmo F, et al. Arterial devices for regional hepatic chemotherapy: transaxillary versus laparotomic access. J Vasc Access 2000;1:93-9.
- Lewis HL, Bloomston M. Hepatic Artery Infusional Chemotherapy. Surg Clin North Am 2016;96:341-55. [Crossref] [PubMed]
- Ensminger WD, Rosowsky A, Raso V, et al. A clinical-pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2'-deoxyuridine and 5-fluorouracil. Cancer Res 1978;38:3784-92. [PubMed]
- Guthoff I, Lotspeich E, Fester C, et al. Hepatic artery infusion using oxaliplatin in combination with 5-fluorouracil, folinic acid and mitomycin C: oxaliplatin pharmacokinetics and feasibility. Anticancer Res 2003;23:5203-8. [PubMed]
- van Riel JM, van Groeningen CJ, Kedde MA, et al. Continuous administration of irinotecan by hepatic arterial infusion: a phase I and pharmacokinetic study. Clin Cancer Res 2002;8:405-12. [PubMed]
- Fiorentini G, Rossi S, Dentico P, et al. Irinotecan hepatic arterial infusion chemotherapy for hepatic metastases from colorectal cancer: a phase II clinical study. Tumori 2003;89:382-4. [Crossref] [PubMed]
- Fiorentini G, Rossi S, Dentico P, et al. Oxaliplatin hepatic arterial infusion chemotherapy for hepatic metastases from colorectal cancer: a phase I-II clinical study. Anticancer Res 2004;24:2093-6. [PubMed]
- Datta J, Narayan RR, Kemeny NE, et al. Role of Hepatic Artery Infusion Chemotherapy in Treatment of Initially Unresectable Colorectal Liver Metastases: A Review. JAMA Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol 1992;10:896-903. [Crossref] [PubMed]
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000;343:905-14. [Crossref] [PubMed]
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644-57; discussion 657-8. [PubMed]
- Basso M, Dadduzio V, Ardito F, et al. Conversion Chemotherapy for Technically Unresectable Colorectal Liver Metastases: A Retrospective, STROBE-Compliant, Single-Center Study Comparing Chemotherapy Alone and Combination Chemotherapy With Cetuximab or Bevacizumab. Medicine (Baltimore) 2016;95:e3722. [Crossref] [PubMed]
- Gruenberger T, Bridgewater J, Chau I, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol 2015;26:702-8. [Crossref] [PubMed]
- Cho M, Gong J, Fakih M. The state of regional therapy in the management of metastatic colorectal cancer to the liver. Expert Rev Anticancer Ther 2016;16:229-45. [Crossref] [PubMed]
- Kemeny N, Daly J, Reichman B, et al. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med 1987;107:459-65. [Crossref] [PubMed]
- Hohn DC, Stagg RJ, Friedman MA, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol 1989;7:1646-54. [Crossref] [PubMed]
- Martin JK Jr, O'Connell MJ, Wieand HS, et al. Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer. A randomized trial. Arch Surg 1990;125:1022-7. [Crossref] [PubMed]
- Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet 2003;361:368-73. [Crossref] [PubMed]
- Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006;24:1395-403. [Crossref] [PubMed]
- Mocellin S, Pilati P, Lise M, et al. Meta-analysis of hepatic arterial infusion for unresectable liver metastases from colorectal cancer: the end of an era? J Clin Oncol 2007;25:5649-54. [Crossref] [PubMed]
- D'Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol 2011;18:1096-103. [Crossref] [PubMed]
- Safi F, Bittner R, Roscher R, et al. Regional chemotherapy for hepatic metastases of colorectal carcinoma (continuous intraarterial versus continuous intraarterial/intravenous therapy). Results of a controlled clinical trial. Cancer 1989;64:379-87. [Crossref] [PubMed]
- Allen-Mersh TG, Glover C, Fordy C, et al. Randomized trial of regional plus systemic fluorinated pyrimidine compared with systemic fluorinated pyrimidine in treatment of colorectal liver metastases. Eur J Surg Oncol 2000;26:468-73. [Crossref] [PubMed]
- Pilati P, Mammano E, Mocellin S, et al. Hepatic arterial infusion for unresectable colorectal liver metastases combined or not with systemic chemotherapy. Anticancer Res 2009;29:4139-44. [PubMed]
- Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2009;27:3465-71. [Crossref] [PubMed]
- Goere D, Deshaies I, de Baere T, et al. Prolonged survival of initially unresectable hepatic colorectal cancer patients treated with hepatic arterial infusion of oxaliplatin followed by radical surgery of metastases. Ann Surg 2010;251:686-91. [Crossref] [PubMed]
- D'Angelica MI, Correa-Gallego C, Paty PB, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg 2015;261:353-60. [Crossref] [PubMed]
- Levi FA, Boige V, Hebbar M, et al. Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann Oncol 2016;27:267-74. [Crossref] [PubMed]
- Lim A, Le Sourd S, Senellart H, et al. Hepatic Arterial Infusion Chemotherapy for Unresectable Liver Metastases of Colorectal Cancer: A Multicenter Retrospective Study. Clin Colorectal Cancer 2017;16:308-15. [Crossref] [PubMed]
- Pak LM, Kemeny NE, Capanu M, et al. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: Long term results and curative potential. J Surg Oncol 2018;117:634-43. [Crossref] [PubMed]
- Chan KM, Wu TH, Cheng CH, et al. Prognostic significance of the number of tumors and aggressive surgical approach in colorectal cancer hepatic metastasis. World J Surg Oncol 2014;12:155. [Crossref] [PubMed]
- Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999;341:2039-48. [Crossref] [PubMed]
- Lorenz M, Muller HH, Schramm H, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen). Ann Surg 1998;228:756-62. [Crossref] [PubMed]
- Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol 2002;20:1499-505. [PubMed]
- Lygidakis NJ, Sgourakis G, Vlachos L, et al. Metastatic liver disease of colorectal origin: the value of locoregional immunochemotherapy combined with systemic chemotherapy following liver resection. Results of a prospective randomized study. Hepatogastroenterology 2001;48:1685-91. [PubMed]
- Nelson R, Freels S. Hepatic artery adjuvant chemotherapy for patients having resection or ablation of colorectal cancer metastatic to the liver. Cochrane Database Syst Rev. 2006.CD003770. [PubMed]
- Kemeny NE, Chou JF, Boucher TM, et al. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol 2016;113:477-84. [Crossref] [PubMed]
- House MG, Kemeny NE, Gonen M, et al. Comparison of adjuvant systemic chemotherapy with or without hepatic arterial infusional chemotherapy after hepatic resection for metastatic colorectal cancer. Ann Surg 2011;254:851-6. [Crossref] [PubMed]
- Groot Koerkamp B, Sadot E, Kemeny NE, et al. Perioperative Hepatic Arterial Infusion Pump Chemotherapy Is Associated With Longer Survival After Resection of Colorectal Liver Metastases: A Propensity Score Analysis. J Clin Oncol 2017;35:1938-44. [Crossref] [PubMed]
- Hamady ZZ, Lodge JP, Welsh FK, et al. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg 2014;259:543-8. [Crossref] [PubMed]
- Buisman FE, Homs MYV, Grunhagen DJ, et al. Adjuvant hepatic arterial infusion pump chemotherapy and resection versus resection alone in patients with low-risk resectable colorectal liver metastases - the multicenter randomized controlled PUMP trial. BMC Cancer 2019;19:327. [Crossref] [PubMed]
- Goere D, Pignon JP, Gelli M, et al. Postoperative hepatic arterial chemotherapy in high-risk patients as adjuvant treatment after resection of colorectal liver metastases - a randomized phase II/III trial - PACHA-01 (NCT02494973). BMC Cancer 2018;18:787. [Crossref] [PubMed]
- McAuliffe JC, Qadan M, D'Angelica MI. Hepatic resection, hepatic arterial infusion pump therapy, and genetic biomarkers in the management of hepatic metastases from colorectal cancer. J Gastrointest Oncol 2015;6:699-708. [PubMed]
- Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011;117:4623-32. [Crossref] [PubMed]
- Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer 2011;128:2075-84. [Crossref] [PubMed]
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073-2087.e3. [Crossref] [PubMed]
- Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J Gastroenterol 2012;18:5171-80. [PubMed]
- Karagkounis G, Torbenson MS, Daniel HD, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer 2013;119:4137-44. [Crossref] [PubMed]
- Yaeger R, Cercek A, Chou JF, et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer 2014;120:2316-24. [Crossref] [PubMed]
- Kemeny NE, Chou JF, Capanu M, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer 2014;120:3965-71. [Crossref] [PubMed]
- Gholami S, Kemeny NE, Boucher TM, et al. Adjuvant Hepatic Artery Infusion Chemotherapy is Associated With Improved Survival Regardless of KRAS Mutation Status in Patients With Resected Colorectal Liver Metastases: A Retrospective Analysis of 674 Patients. Ann Surg 2019. [Epub ahead of print].


