Status of hepatocellular carcinoma in South Korea
Incidence and mortality of hepatocellular carcinoma (HCC) in South Korea
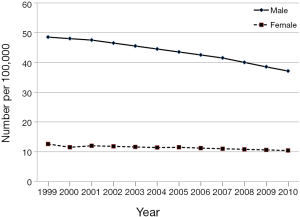
According to National Cancer Information Center (NCIC, www.cancer.go.kr) in Korea, 15,921 cases (22.9 cases per 100,000 population) were estimated to be diagnosed with liver cancer, accounting for 7.9% of all cancers diagnosed in 2010. Liver cancer ranks the 5th most common cancer after thyroid cancer, stomach cancer, colon cancer and lung cancer in South Korea. It is the 4th most common cancer in Korean men and 6th most common cancer in Korean women. However, the incidences of liver cancer among Korean men and women have been declining from 1999 (48.5/100,000 and 12.6/100,000) to 2010 (37.1/100,000 and 10.4/100,000) (Figure 1). The reason for the declining incidence appears secondary to decreased hepatitis B virus (HBV) infection, which is the leading risk factor for HCC. With the implementation of national HBV vaccination program for infants since 1983 in South Korea, HBV infection has become preventable, the incidence of HCC has been dramatically decreased. In contrast, the incidences of HCC in recent decades have increased in Western countries due to the rise in hepatitis C virus (HCV) infection through continued transmission by drug abusers (1). In addition, recent report showed that obesity is positively linked with the developing HCC paradigm in Western countries (2,3). Accordingly, the geographic variations for the incidence of HCC can be explained by the regional differences in the prevalence of risk factors for HCC.
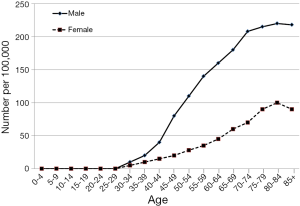
The HCC incidence rates in Korea are more than twice higher for males than females. The age-standardized (or adjusted) incidence rate per 100,000 population for liver cancer in 2010 is shown in Figure 2. The incidence rate continues to increase with age and is highest between the age of 80 to 85 both in man and women. However, along with the recent trend towards increased incidence of oral cancer among young adults, HCC appears be the most common cancer in their forties.
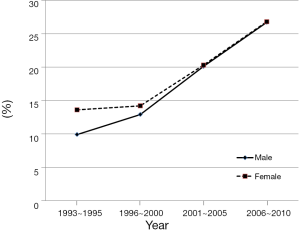
According to mortality data from Korean Statistical Information Service (KOSIS, http://kosis.kr) in 2011, the liver cancer death rate was reported as high as 32.8/100,000 for man and 10.9/100,000 for women. The number of death from liver cancer in Korea increased gradually from 2003 (9,500/100,000) to 2011 (10,946/100,000). Despite recent advances in the treatment of HCC, many patients cannot be cured due to the advanced stage of disease at the time of diagnosis in Korea. Accordingly, the 5-year survival rate in Korea is relatively lower than other cancers. Nonetheless, it has been gradually improved from the early 1990s (10.7%) to the late 2000s (26.7%) (Figure 3). The reason for the improvement in 5-year survival rates can be attributed that early detection of HCC becomes possible by well-established surveillance program in high-risk population for HCC in Korea.
Risk factors
Hepatocarcinogenesis has been proposed as a progressive multistep process evolving from chronic inflammation and cirrhosis to HCC. In general, the risk factors for developing HCC are well known in comparison with other cancers. In Korea, among the major risk factors, chronic HBV infection has been reported as the most common risk factor for developing HCC and approximately 65% to 75% of HCC cases were positive for hepatitis B surface antigen (HBsAg) (4,5). Next, chronic HCV infection was the 2nd common cause for developing HCC, estimated to be accountable for 11.2% to 13.2% of all HCC cases (4,5). Other, probably compound, risk factors included heavy alcohol consumption, cigarette smoking and family history of HCC (5,6). Specifically, Aflatoxin B1, a mycotoxin produced by the Aspergillus fungus, can cause HCC in non-cirrhotic livers (7). In addition, other less common or very rare risk factors observed in Korea include primary biliary cirrhosis, hereditary hemochromatosis, α1-1 anti-trypsin deficiency, Wilson’s disease, echinococcosis, and schistosomiases (8).
The reason for high incidence of HCC in Korea results from a large number of HBV carrier among general population, approximately accounting for 5% of the general Korean population (9). Moreover, a previous study by Jee et al. in Korea reported that, while cigarette smoking, heavy alcohol consumption, and HBsAg were independently associated with increased risk of mortality from HCC, they appeared not interact synergistically (6). Regarding the association between the incidence of HCC and family history of HCC, Park et al. reported a retrospective analysis in a large group of HCC patients (5). Of the 2,242 patients diagnosed with HCC, 165 (7.4%) had a positive family history of HCC. Among 1,713 HCC patients with HBV infection, 136 patients had a positive family history. The number of patients under 45 years of age with HBV infection and positive family history was 26 (19.1%), whereas those out of 1,577 patients with negative family history was 197 (12.5%), suggesting that a positive family history may be associated with earlier development of HCC in the Korean population.
Diagnosis and staging of HCC
Diagnostic investigations for HCC comprise three processes including blood tests (serum tumor markers), imaging procedures and the histologic confirmation. In principle, the diagnosis of HCC should be based on histologic confirmation. However, percutaneous liver biopsy has several drawbacks, such as incorrect tumor targeting for the small lesion and the potential for tumor seeding through the needle track. Accordingly, non-invasive methods like tumor markers or imaging studies are commonly used for the clinical diagnosis of HCC. However, a histologic confirmation of HCC is mandated for most of prospective clinical studies especially with investigational new drugs or target agents.
Currently used tumor markers for the diagnosis of HCC have included α-fetoprotein (AFP), des-γ-carboxy prothrombin (DCP) or protein induced by vitamin K absence or antagonist (PIVKA-II) and a fucosylated variant of the AFP glycoprotein which has a high affinity to the sugar chain of Lens culinaris (AFP-L3) (10). Previous studies have shown that the sensitivity and specificity of AFP for the diagnosis of HCC are 41-65% and 80-94%, respectively, with a cutoff value of 20 ng/mL (11). This variability in the sensitivity among different studies is thought be due to several factors including study design (retrospective vs. prospective study), insufficient sample, size, etiologic factors of HCC, race and the different cutoff values used for AFP (10). DCP as a new diagnostic marker for HCC was initially reported by Liebman et al. (12). Recent reports have described the sensitivities and specificities of DCP for the diagnosis of HCC ranging from 44.3-92% and 93-97%, respectively (13-15). The comparative study of AFP, DCP and AFP-L3 for diagnostic value showed that DCP was significantly better than total AFP or AFP-L3 in differentiating HCC from cirrhosis, with a sensitivity of 86% and specificity of 93% (15). Although AFP-L3 was approved as a diagnostic tumor marker for HCC, it is not widely used in the clinical practice because of its difficulties in procedure and interpretation. In Korea, AFP and PIVKA-II are commonly used as a diagnostic marker at the present time and the use of additional DCP has been continuously increasing.
Meanwhile, with the advancement in the imaging techniques, such as multiphasic spiral computed tomography (CT), dynamic magnetic resonance imaging (MRI) showing higher sensitivity and specificity, the diagnostic value of currently used serum tumor markers has been enfeebled and their diagnostic values have recently been with a significant controversy worldwide. The current practice guidelines established by the American Association for the Study of Liver Diseases (AASLD) (16) and the Asia-Pacific Association for the Study of the Liver (APASL) (17) as well as by the European Association for the Study of the Liver (EASL) (18) do not recommend AFP test for the surveillance or diagnosis of HCC. While the AASLD guidelines recommended that the diagnosis of HCC should be based on imaging techniques and/or biopsy, the APASL guidelines recommended dynamic imaging techniques regardless of the tumor size and AFP (17). However, Korean Liver Cancer Study Group (KLCSG) guidelines established in 2009 (19) included AFP level for the diagnosis of HCC. If the serum AFP level is ≥200 ng/mL in a high-risk patient, typical characteristics of HCC in either dynamic contrast enhancement CT or dynamic contrast enhancement MRI may satisfy the diagnosis of HCC. If the serum AFP level is <200 ng/mL, two or more positive findings of (I) dynamic contrast enhancement CT; (II) dynamic contrast enhancement MRI; or (III) hepatic arterial angiography are necessary to make the diagnosis of HCC. A histologic confirmation is not necessary to establish the diagnosis of HCC in Korea if certain imaging criteria are met. When the diagnosis of HCC by a radiologic or histologic examination for the liver nodule less than 1 cm in diameter cannot be verified, repeated periodical examinations of serum tumor markers and ultrasonography at 3- to 6-month interval have been recommended.
In general, the treatment options and prognosis of HCC have been mainly determined according to the tumor stage, liver dysfunction and performance status. To date, several staging systems in different countries have been proposed for HCC, including Barcelona Clinic Liver Cancer (BCLC) (20), TNM (21), Cancer of the Liver Italian Program (CLIP) (22), Chinese University Prognostic Index (CUPI) (23), Japan Integrated Staging Score (JIS score) (24), the Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire Prognostic Classification (GETCH) (25). However, there is no universally accepted consensus among different staging systems for HCC at the present time. In Korea, modified International Union Against Cancer (UICC) staging system (26) and the BCLC staging system have been commonly used as a staging system for HCC. Nonetheless, greater portions of HCC patients are diagnosed at advanced stage. For example, we analyzed the tumor stage at the time of diagnosis in 2,241 HCC patients who were treated at a single tertiary academic hospital in Korea over 18-year period (5). By the modified UICC staging classification, only 185 patients (8.3%) were diagnosed at stage I, followed by 655 (29.2%) patients at stage II, 648 (28.9%) patients at stage III, and 753 (33.6%) patients at stage IV. More than half of the HCC patients (62.5%) were diagnosed at advanced stages, including stages III and IV, in this retrospective analysis. Meanwhile, according to the registry data from the Korean Liver Cancer Study Group (www.klcsg.or.kr), the stage distributions at initial diagnoses were 10.7%, 43.4%, 27.7%, and 18.2% for stages I, II, III, and IV (Modified TNM Stage by LCSGJ), respectively, for 4,521 patients from 32 Korean hospitals randomly sampled from 31,521 patients registered with the liver cancer during a 3-year period [2003-2005]. Accordingly, only a small proportion of the HCC patients can receive curative treatments including surgical resection and liver transplantation (LT) in South Korea.
Screening and surveillance program for early detection for HCC
Screening refers to the use of simple and inexpensive test across healthy population in order to identify individuals who are likely or unlikely to have a cancer whereas surveillance defines continuous monitoring of disease occurrence using the screening test until a cancer appears. Because the surveillance program in the high-risk patients of HCC allows for the detection of HCC at early stage, a greater portion of patients are suitable for potentially curative treatments including liver resection or LT. AFP, most commonly used tumor marker, and liver ultrasonography have been employed for the surveillance test of HCC in Korea. The national surveillance program for HCC was established in 2003, in which repeated applications of these screening tests at 6-month intervals have been recommended in patients at high risk for developing HCC, such as men and women older than 40 years of age with positive HBsAg, anti-HCV Ab or underlying liver cirrhosis in Korea. A 15-year prospective study in Korea indicated an improved survival of HCC patients with surveillance interval ≤6 months compared with >6 months (27).
Treatment and management of HCC
Similar to rest of the world, treatment of HCC in South Korea has been remarkably advanced over the last 20 years. The treatment of HCC can be classified into two categories: curative treatment and palliative treatment. Curative treatments include LT, surgical resection and radiofrequency ablation (RFA) while palliative treatments include transarterial chemoembolization (TACE), radioembolization using radionuclide Yttrium-90, systemic therapy, radiation therapy and molecular target therapy. The treatment option for HCC depends on the tumor stage and liver dysfunction.
LT, in theory, is the most ideal therapeutic modality because it can cure underlying liver cirrhosis as well as removing the tumor in HCC patients with liver cirrhosis. However, only a small proportion of HCC patients can undergo LT because of the donor shortage, high cost and advanced tumor stage at the time of diagnosis. In general, a precise tumor assessment in HCC patients according to Milan criteria, including single tumor less than 5 cm or two to three tumors with the largest being less than 3 cm in the absence of portal vein invasion and extrahepatic metastasis (28), is required prior to LT. Although the first case of LT was performed in a 14-year old boy with Wilsons’ disease in 1988, cumulative experience on LTs performed in Korea before 2000 is not available. According to the Annual Report of the Transplantation published by the Ministry of Health and Welfare, ROK [2013], there were a total of 9,380 cases of LT in Korea during a period of 2000-2012 (29). The majority (79.6%; 7,468 cases) of LT had been performed with living donors, while 1,912 cases (20.4%) were by donors with brain death. During the year of 2012, there were a total of 1,260 patients underwent LT in Korea; 897 with living donors and 363 with brain death donors. The 1-year and 5-year survival rates were 88.5% and 80.0% respectively after living donor LTs, and 77.3% and 69.5% respectively for LTs with brain death donors.
Although surgical resection has been accepted as a treatment modality of choice for HCC, it has a limited role since the tumors in most patients are unresectable due to variety of factors including poor hepatic reserve, multifocality of HCC or inability to obtain an optimal tumor-free margin. Furthermore, recurrent HCCs are frequently found in the residual liver within a short period of time after surgical resection. Nevertheless, the possibility of surgical resection should be considered as a therapeutic option when the initial clinical diagnosis of HCC is made in a patient in Korea. In addition, surgical resection is also considered for HCC patients beyond Milan criteria. A retrospective review of a single Korean institution experience on LT vs. surgical resection reported a statistically insignificant overall survival but a significant (P=0.002) recurrence-free survival for LT (30). However, after recurrence, surgical resection had a better survival than LT. Depending upon the location and size of the HCC, surgical resection of HCC lesion has been performed via laparoscopic approach in selected patients.
Loco-regional treatments for HCC are important alternatives to curative LT or surgical resection. Among them, RFA is accepted as the most popular technique showing excellent local tumor control and acceptable morbidity as adopted in practice guidelines in North America, Europe and Japan (31). The overall survival after RFA is comparable to after surgical resection in a selected group of patients with smaller (<3 cm) tumors. RFA has been frequently used in Korea as well. Choi et al. reported single institution experience of percutaneous RFA in 570 patients with 674 early-stage HCCs as a first-line treatment option (32). The primary technique effectiveness rate was 96.7% (652 of 674). The cumulative rates of local tumor progression at one, two, and three years were 8.1%, 10.9%, and 11.8%, respectively. The cumulative survival rates at one, two, three, four, and five years were 95.2%, 82.9%, 69.5%, 60.8%, and 58.0%, respectively. Patients with Child-Pugh class A cirrhosis, of younger age (≤58 years), or having lower AFP level (≤100 µg/L) demonstrated better survival results (P<0.05).
Although TACE is considered a palliative therapeutic modality for HCC patients, >50% improvement in the 5-year survival rates has been reported (33). While it is recommended that TACE should be used for intermediate stage according to BCLC staging system, many cases with various stages under specific condition have been treated with TACE in real-life clinical practice in Korea. TACE was introduced as a palliative therapy of HCC in mid-1980s and has been adopted as the most commonly used loco-regional therapeutic modality for HCC in Korea. Earlier 6-year experience on 1,067 HCC patients at a single Korean institution reported 1-, 2-, 3-, 4-, and 5-year survival rates of 60.6%, 42.3%, 29.1%, 23.7%, and 14.7%, respectively (34). For 432 patients with tumors ≤5 cm in diameter, 1-year survival rate and median survival were 77.7% and 33 months, respectively. Significant prognostic factors included size and type of the tumor, portal vein invasion, and Child-Pugh classification. A prospective single institution cohort study comparing TACE to surgical resection on 182 patients with operable HCC (Child-Pugh class A and UICC stage T1-3N0M0) reported that survival rate of surgical resection group was comparable to that of TACE group (P=0.0596) (35). Recently, in order to maximize the therapeutic efficacy of TACE, doxorubicin-loaded drug-eluting beads (DC beads) have been developed to deliver higher doses of the chemotherapeutic agent and to prolong contact time with the tumor. We recently reported the comparative study in the efficacy and safety between DC bead TACE and conventional TACE (cTACE) (36). The time to progression was significantly better in the DC bead® group than in the cTACE group (11.7 and 7.6 months, respectively). Subgroup analysis showed that DC bead® treatment resulted in a significantly better treatment response and longer time to progression than cTACE (P<0.001 and 0.038, respectively) in intermediate-stage HCC.
There are limited options for the systemic treatment of patients with advanced or metastatic HCC. Although systemic anti-cancer chemotherapeutic drugs have been applied for these patients, data demonstrating their efficacy in patients are limited. Therefore, novel molecularly targeted therapy directing key signaling pathway has been explored based on the molecular mechanism underlying various HCC. Sorafenib is a multikinase inhibitor targeting Raf, vascular endothelial growth factor receptor (VEGFR) linked in angiogenesis signaling pathways (37). The results from two independent (one in Europe and US and another in Asia-Pacific region) phase III studies with sorafenib in chemotherapy-naive unresectable advanced HCC demonstrated advantages in OS compared to placebo (10.7 vs. 7.9 months in Europe/US study and 6.5 vs. 4.2 months in Asia-Pacific study) (37,38). Subsequently, sorafenib has been approved by regulatory authorities and used worldwide as a standard agent for the systemic therapy of advanced HCC. While sorafenib was approved by the Korean Food and Drug Administration for the treatment of advanced/metastatic HCC in March 18, 2008, the agent became fully reimbursable by the national insurance system only early this year.
Future perspectives
In order to overcome the poor prognosis of HCC patients, it is absolutely imperative to recognize the risk factors for developing HCC. Chronic HBV and HCV infections account for 54.4% and 31.1% of all HCC cases occurred globally, and thus both HBV and HCV infection should be prevented or eradicated using effective immunization or anti-viral agents, respectively. Therefore, the main goal for the treatment of chronic HBV infection should be the eradication of HBV or at least suppression of viral replication. Among several viral markers for chronic HBV infection, both HBV DNA and HBsAg levels were shown to be associated with HCC development (39). Since the introduction of nationwide HBV vaccination program in 1983 in Korea, the incidence of HBV in neonates is now estimated less than 0.5%, which is predicted to be nil by 2030. With adoption of successful HBV vaccination program, it is anticipated that HBV related HCC incidence will be proportionally decreasing and Non-B (HCV or NBNC) related HCC will be proportionally increasing in Korea. Therefore, new strategy to screen and control of non-B related chronic liver diseases will be necessary to control HCC in Korea and continued education and training for health-care providers for screening and surveillance as well as management for HCC are indicated. The consensus-based treatment algorithm (e.g., BCLC algorithms) may not always be optimal for Korean HCC patients in clinical practice. Therefore, the development of a consensus HCC management algorithm optimal for Korean HCC patients based on the updated data is desirable. In the future, evidence-lacking parts of the algorithm should be improved through prospective studies.
Besides having a liver disease, patients with HCC require different management strategies for their illness, delivered by various specialists. Furthermore, the presence of confounding factors means that no single treatment strategy can be applied to all patients, and therefore therapy may be tailored to each patient’s needs. In particular, since no specific guidelines exist to ensure the best care for HCC patients with cirrhosis, it is crucial to establish a close cooperation between these specialists, together with a cautious approach to decision making for these patients. Of note, specialists in pathology, gastroenterology, hepatology, hepatobiliary surgery, transplant surgery, interventional and diagnostic radiology, medical oncology, radiation oncology and nuclear medicine are involved in the management of HCC patients. In Korea, liver resection has been performed by a liver surgeon and regional intrahepatic therapeutic modalities (TACE, RFA, etc.) have been achieved by either diagnostic or interventional radiologists. Until recently, medical therapy of HCC using anti-cancer agents in Korea, including TACE, intrahepatic/regional and/or systemic chemotherapies, has been predominantly managed by gastroenterologists (hepatologists) and interventional radiologists. Similar to other malignant diseases, with rapid advances in medical and oncologic sciences, integrated multidisciplinary team approach should be implemented for optimal management of HCC patients with improved clinical outcomes.
The development of selective targeted drugs, especially Sorafenib (Nexavar®), represented a major progress in the treatment of advanced HCC with well-preserved liver function. However, since the therapeutic benefit of sorafenib monotherapy is rather limited, continued exploration of new novel agents for HCC is obviously indicated. There are a number of clinical studies with new agents currently ongoing or planned in Korea. The patient accrual has been completed for a phase 2b randomized trial of JX-594 (Vaccinia GM-CSF/TK-deactivated virus) in patients with advanced HCC who have failed sorafenib conducted at a limited number of Korean institutes. Several Korean institutions are actively participating in a randomized, double-blind, multicenter, phase 3 international study evaluating ADI-PEG 20 (Arginine deprivation agent) as a second-line therapy of metastatic HCC. Another phase 3 randomized, double-blind, international trial to evaluate Cabozantinib (XL184; oral tyrosine kinase inhibitor with potent activity against MET and VEGFR2 signaling) is ready to be started in Korea. A phase I/II study of a combination of Sorafenib and Resminostat [a hydroxamic acid with an inhibitory effect on histone deacetylases (HDACs)] in patients with systemic therapy naïve metastatic HCC has been designed and plans to be conducted at limited Korean and Japan institutions. Proposals for clinical trials with Enzalutamide (Androgen Receptor inhibitor) as well as other agents (e.g., ASP5878) are currently under discussion.
In conclusion, chronic HBV infection remains to be the most important risk factor responsible for the development of HCC in Korea. It is essential that the nationwide surveillance program for HCC should be effectively executed in high-risk patients (e.g., HBV cirrhosis or carrier) for developing HCC. In addition, extensive research should be explored in order to understand molecular pathways of HCC, and to identify new biomarkers and candidate target molecules. Optimal application of multidisciplinary team approach and active involvement in clinical studies with new agents in HCC patients are critically important not only for the management of HCC patients but also for the improvement in natural history and therapeutic outcomes of HCC patients in the future.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012-001941).
Disclosure: The authors declare no conflict of interest.
References
- el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis 2001;5:87-107. [PubMed]
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485-91. [PubMed]
- Bosetti C, Levi F, Boffetta P, et al. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology 2008;48:137-45. [PubMed]
- Shin HR, Lee CU, Park HJ, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol 1996;25:933-40. [PubMed]
- Park CH, Jeong SH, Yim HW, et al. Family history influences the early onset of hepatocellular carcinoma. World J Gastroenterol 2012;18:2661-7. [PubMed]
- Jee SH, Ohrr H, Sull JW, et al. Cigarette smoking, alcohol drinking, hepatitis B, and risk for hepatocellular carcinoma in Korea. J Natl Cancer Inst 2004;96:1851-6. [PubMed]
- Abdel-Wahab M, Mostafa M, Sabry M, et al. Aflatoxins as a risk factor for hepatocellular carcinoma in Egypt, Mansoura Gastroenterology Center study. Hepatogastroenterology 2008;55:1754-9. [PubMed]
- Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist 2010;15 Suppl 4:14-22. [PubMed]
- Lee MS, Kim DH, Kim H, et al. Hepatitis B vaccination and reduced risk of primary liver cancer among male adults: a cohort study in Korea. Int J Epidemiol 1998;27:316-9. [PubMed]
- Yoon SK. Recent advances in tumor markers of human hepatocellular carcinoma. Intervirology 2008;51 Suppl 1:34-41. [PubMed]
- Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med 2003;139:46-50. [PubMed]
- Liebman HA, Furie BC, Tong MJ, et al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med 1984;310:1427-31. [PubMed]
- Marrero JA, Su GL, Wei W, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology 2003;37:1114-21. [PubMed]
- Nakamura S, Nouso K, Sakaguchi K, et al. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am J Gastroenterol 2006;101:2038-43. [PubMed]
- Volk ML, Hernandez JC, Su GL, et al. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark 2007;3:79-87. [PubMed]
- Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [PubMed]
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74. [PubMed]
- European Association for Study of Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer 2012;48:599-641. [PubMed]
- Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol 2009;15:391-423. [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [PubMed]
- Lei HJ, Chau GY, Lui WY, et al. Prognostic value and clinical relevance of the 6th Edition 2002 American Joint Committee on Cancer staging system in patients with resectable hepatocellular carcinoma. J Am Coll Surg 2006;203:426-35.
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751-5. [PubMed]
- Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer 2002;94:1760-9. [PubMed]
- Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 2003;38:207-15. [PubMed]
- Chevret S, Trinchet JC, Mathieu D, et al. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol 1999;31:133-41. [PubMed]
- Ueno S, Tanabe G, Nuruki K, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res 2002;24:395-403.
- Han KH. Survival of hepatocellular carcinoma patients may be improved in surveillance interval not more than 6 months compared with more than 6 months: a 15-year prospective study. J Clin Gastroenterol 2013;47:538-44. [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [PubMed]
- The Annual Report of the Transplantation by the Ministry of Health and Welfare, ROK, 2013.
- Lee KK, Kim DG, Moon IS, et al. Liver transplantation versus liver resection for the treatment of hepatocellular carcinoma. J Surg Oncol 2010;101:47-53. [PubMed]
- Rhim H, Lim HK, Choi D. Current status of radiofrequency ablation of hepatocellular carcinoma. World J Gastrointest Surg 2010;2:128-36. [PubMed]
- Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol 2007;17:684-92. [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [PubMed]
- Park JH, Chung JW, Lee SK, et al. Chemoembolization of hepatocellular carcinoma: long-term survival and prognostic factors. J Korean Radiol Soc 1996;35:315-23.
- Lee HS, Kim KM, Yoon JH, et al. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus-endemic area: a prospective cohort study. J Clin Oncol 2002;20:4459-65. [PubMed]
- Song MJ, Chun HJ. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol 2012;57:1244-50. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [PubMed]
- Kao JH. Risk stratification for HBV-related HCC. The 51th Annual Meeting of Japan Society of Clincal Oncology, October 24-26, 2013 (IS1-2).