
Long term control stereotactic body radiotherapy (SBRT) for oligometastatic colorectal cancer: a single center study
Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in Europe and the second cause of death both in Europe and worldwide (1). Early detection of CRC is associated with 5-year survival rates as high as 93% in patients staged T1/2N0M0, but 25% of the patients have metastases at diagnosis and 50% will develop them during the evolution of the disease.
Systemic therapy is the gold standard of care in stage IV CRC with median OS of 30 months approximately (2). Colorectal tumors frequently have solitary or oligometastases (1–5 lesions), being the liver and lung the most affected organs. Surgical treatment of resectable disease in the metastatic setting has increased OS (3-5). Different alternatives to surgery in non-resectable patients have been explored, such as radiofrequency ablation (RFA) and radiotherapy (6,7). Technological advances in the radiotherapy landscape, have allowed the introduction of stereotactic body radiotherapy (SBRT). SBRT involves a very accurate delivery of a high radiation dose in a small number of fractions to a target with narrow margins. The limited volume of normal tissue exposed to radiation is potentially attractive, and the high dose per fraction, with low number of treatment fractions is appealing because of the potential for immune effects (8). The use of SBRT has reported promising results allowing local control up to 90% at 2 years (9). However, the optimal time of administration is unknown. It is necessary to identify prognostic factors that can be useful to select those patients who can benefit from this treatment.
The aim of this study is to assess the efficacy and toxicity profile of SBRT as a treatment modality in a cohort of patients with metastatic CRC.
Methods
Study population and ethics
Retrospective study of all consecutive patients with diagnosis of metastatic CRC treated with SBRT on metastatic lesions in different locations between February 2012 and August 2016 at the General University Hospital of Valencia. The variables collected in the study were age, sex, location of the primary tumor, date of surgery, stage of the disease at diagnosis (TNM 8th), mutational status of genes RAS, location and number of metastases and type of relapse. Patients could have previously received treatment both primary and metastatic disease.
Baseline disease extension and tumor response evaluation was carried out by CT scans. Tumor response was evaluated according to RECIST 1.1 criteria.
Follow-up patient parameters (time of relapse and exitus) were recorded since time of study inclusion until 15 July 2018. The study was approved by The Clinical Research Ethics Committee of General University Hospital of Valencia, in accordance with the Declaration of Helsinki, the Good Clinical Practices and local ethical and legal requirements (Spanish laws). This study complied with all applicable regulations for human participant studies. All authors reviewed and approved the final manuscript.
SBRT treatment
All patients underwent TAC planning gating with WING-BOARD immobilization system and abdominal compression system for respiratory control (Dumpening) that are also used during treatment. The treatment volume (ITV) is generated with the MIM contouring system and the planning is done using Pinnacle.
We define the PTV as the ITV generated from the union of the different GTVs of the TAC 4D plus a volumetric expansion of 5 mm. Dose was prescribed to the isocenter and the PTV was covered by at least 95% of the prescription dose. The patients are treated in a TRUEBEAM linear accelerator, positioning themselves with KV and subsequent verification CONEBEAM, which is performed pre- and post-treatment. SBRT was delivered using conformal arcs or multiple fixed no-coplanar beams, shaped with multileaf collimators, with 6Mv FFF photons. The dose per fraction and total dose were determined using the dose volume histogram (HDV) of the organs at risk.
The fraction dose, in lung metastases, is established following a risk-adapted fractionation of the Dutch Senan group (3×20 Gy in small tumors not close to the costal wall or central location, 5×12 Gy in larger tumors and/or close to the costal wall and 8×7.5 Gy in central tumors location—located less than 2 cm from the main tracheobronchial system). In case of liver or lymph node metastases the dose is established based on the consensus established by SEOR in 2014 with the objective of obtaining total doses with biologically effective dose (BED) 10 Gy >100 Gy. Toxicity was evaluated according to RTOG criteria.
The patients have a follow-up weekly during the treatment and image scans were performed every 3 months during the first year.
Statistical methods
All statistical analyses were performed using the SPSS statistical package, version 22 (SPSS, Inc., Chicago, IL, USA). A descriptive statistics analysis including absolute and relative frequencies for categorical variables was performed. A logistic regression multivariate analysis was performed introducing the variables which were significant in the univariate analysis in the model. The survival curve was estimated using the Kaplan-Meier method and compared using the log-rank test. Statistical survival analyzes were performed using the Kaplan-Meier method and the log-rank statistic. The SLP in months was calculated taking into account the date of the end of treatment with SBRT until the date of the tumor recurrence or the last patient follow-up and the overall survival (OS) taking into account the date of surgery of the primary tumor, by means of the following formula: 12 × (End Date − Start Date)/365.25. The multivariate analysis to evaluate the independent prognostic values was performed using the Cox regression method. For this, all variables that were significant in the univariate analysis and those in which the P value was close to statistical significance were included.
All reported P values were the result of two-sided tests, with P<0.05 considered statistically significant.
Results
Patient characteristics
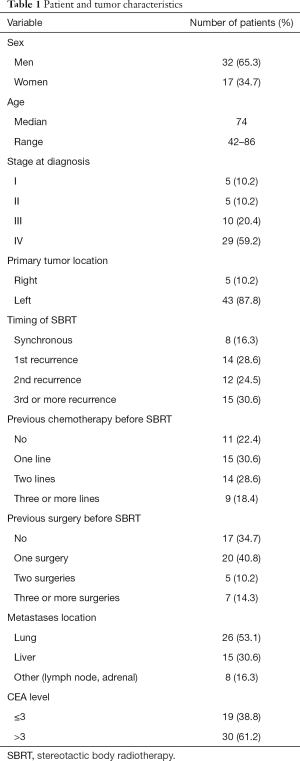
A total of 49 patients were included in the study (65.3% men). The average age was 70 years. The main patient and tumor characteristics are summarized in Table 1. It should be note that only 10% of primary tumors were located in the right colon. Sixty percent of patients were metastatic at diagnosis. The population was highly pretreated with almost 50% of the patients had received 2 or more previous ChT lines.
Full table
Sixty-five point two percent of patients had undergone surgery for metastatic disease prior to treatment with SBRT. Regarding the surgery of the metastases, 65% had been operated in some occasion, with 10% of the patients operated 2 or more times. The main location of the lesions treated with SBRT was pulmonary (53.1%).
Treatment and toxicity
The total dose received in the treatment of SBRT was 45 Gy in 57.4% of the cases (fractionation of 1,500 cGy/session, 3 sessions, BED 93.8 Gy). Twenty-four point five percent received a dose of 60 Gy (fractionation of 2,000 cGy/session, 3 sessions, BED 2,000 Gy). The 18.5% received a total dose between 30 and 40 Gy, and 1.9% a dose of 50 Gy.
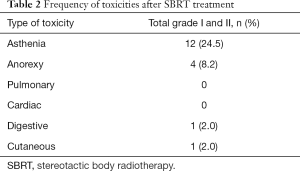
The treatment was well tolerated. The most frequent adverse effects are described in Table 2. No patient had grade 3 or 4 adverse effects.
Full table
OS and progression free survival
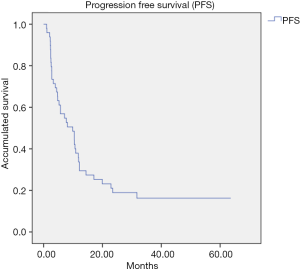
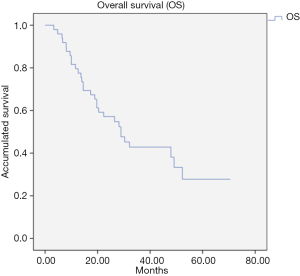
The median follow-up was 26.1 months. The PFS after treatment with SBRT was 9.9 months (95% CI: 4.64–15.1) (Figure 1) and the median OS was 28.9 months (95% CI: 19.0–38.7) (Figure 2). No relapses were observed in 20% of the patients after SBRT. In this subgroup of patients, the median PFS was 41.82 months (95% CI: 24.83–58.81 months).
Factors affecting the treatment outcomes
The univariate survival analysis showed a lower PFS in female (P value =0.05), higher tumor stage (P value =0.054), age more than 70 years (P value =0.012) and previous treatment with chemotherapy (P value =0.07). In multivariate analysis, no factor showed significant influence on PFS.
The univariate survival analysis showed a lower OS in female (P value =0.020), RAS mutated tumors (P value =0.06), right colon tumors (P value =0.014) and higher tumor stage (III–IV vs. I–II) (P value <0.053). Univariate analysis of prognostic factors associated with OS was shown in Table 3.
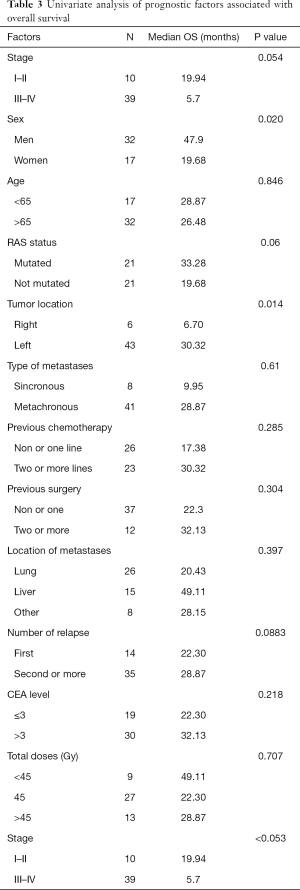
Full table
In multivariate analysis, right colon [HR 16.53 (95% CI: 3.11–87.87), P value 0.001] and higher tumor stage (III–IV) [HR 12.30 (95% CI: 2.10–71.92), P value 0.005] showed a lower OS. No impact on OS was observed regarding sex, RAS status or location of metastases.
Discussion
SBRT is a minimally invasive radiation technology that can provide a large dose of highly focused ionizing radiation to the target tumor and reduce normal tissue toxicity. For CRC, some series reported 5-year survival rate of 52% for patients who have one metastasectomy for pulmonary metastasis and 57.9% for patients who have a second surgery (10). However, there are patients in whom surgery is not indicated due mainly to previous interventions, localization of the disease or comorbidities (11-13). In this scenery, it is necessary to develop new local treatment modalities. SBRT as a radical treatment for oligometastatic disease has emerged as a treatment well tolerated by patients, with high rates of local control and that prolong survival. In retrospective series SBRT offers local control (LC) up to 90% at 2 years (14,15).
Several authors have reported a survival benefit with SBRT in oligometastatic disease. So far the liver location is the best studied with both retrospective and prospective studies. OS have been described that vary from 50% to 100% per year, from 32% to 91% at 2 years and from 60% to 85% at 3 years (7,9,11,12). Lung metastases from retrospective studies show a local control of 77% a year and 67% at 3 years with OS data of 64% to 73% at 2 years (14-17).
According to these results, the median OS of the subgroup of liver metastasis and pulmonary metastases of our study was 49.11 (95% CI: 7.8–90.3) and 20.43 (95% CI: 13.2–27.6) months respectively. The results of the cohort including 16.3% of metastases in other locations showed a median OS of 28.9 (95% CI: 19.0–38.7) months. This benefit in survival is clinically relevant considering that it is a population that is generally very pre-treated, both with previous systemic and local treatment. At the same time, the 10-month median PFS obtained allows patients to have long periods of time without receiving systematic treatment, thus decreasing toxicity and improving their quality of life.
It should be noted that 20% of patients did not relapse after treatment with SBRT during the follow-up of the study, opening the door for an important percentage of patients to obtain a lasting control of the disease.
In all analyzed studies, SBRT show a very low rate toxicity, without adverse effects grade 3–4 (16-19), as we have also observed in our study. The most frequent side effect was asthenia (present in 24.5% of patients), being grade I and II. As we said before, our series was composed of a population that had received many previous treatments, both chemotherapy and surgery and that the good tolerability of the SBRT allows lengthening survival while maintaining the quality of life, a very important factor in this patient subgroup.
Some authors have suggested a greater benefit in the local control of the disease and a good survival associated with high doses of treatment and higher BED (11,18,19). However, the multivariate analysis of our series has not shown a correlation of any of these parameters with survival. On the other hand, it is difficult to establish an ideal treatment dose for different locations due to low unification of published results.
This study has some limitations due to the small sample size, the retrospective design, and the heterogeneity of the cohort. Future large and prospective studies and unified reporting protocol are necessary to establish the role of SBRT in the treatment of oligometastatic disease of CRC.
In conclusion, SBRT can contribute to the local control of the disease and increase the survival of patients with oligometastatic CRC. It is important to discuss each case within a multidisciplinary team to establish the optimal therapeutic sequence with systemic treatments and other local treatments.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco.2020.01.10). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by The Clinical Research Ethics Committee of General University Hospital of Valencia, in accordance with the Declaration of Helsinki, the Good Clinical Practices and local ethical and legal requirements (Spanish laws). This study complied with all applicable regulations for human participant studies.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Wei AC, Greig PD, Grant D, et al. Survival After Hepatic Resection for Colorectal Metastases: A 10-Year Experience. Ann Surg Oncol 2006;13:668-76. [Crossref] [PubMed]
- Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg 2004;240:438-47. [Crossref] [PubMed]
- Small R, Lubezky N, Ben-Haim M. Current controversies in the surgical management of colorectal cancer metastases to the liver. Isr Med Assoc J 2007;9:742-7. [PubMed]
- Ruers T, Van Coevorden F, Punt CJ, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst 2017. [Crossref] [PubMed]
- Lo SS, Fakiris AJ. Stereotactic body radiation therapy for oligometastases. Expert Rev Anticancer Ther 2009;9:621-35. [Crossref] [PubMed]
- Coleman CN, Lawrence TS, Kirsch DG. Enhancing the efficacy of radiation therapy: premises, promises, and practicality. J Clin Oncol 2014;32:2832-5. [Crossref] [PubMed]
- Kobiela J, Spychalski P, Marvaso G, et al. Ablative stereotactic radiotherapy for oligometastatic colorectal cancer: Systematic review. Crit Rev Oncol Hematol 2018;129:91-101. [Crossref] [PubMed]
- Salah S, Watanabe K, Park JS, et al. Repeated resection of colorectal cancer pulmonary oligometastases: pooled analysis and prognostic assessment. Ann Surg Oncol 2013;20:1955-61. [Crossref] [PubMed]
- Joo JH, Park J, Kim JC, et al. Local Control Outcomes Using Stereotactic Body Radiation Therapy for Liver Metastases From Colorectal Cancer. Int J Radiat Oncol Biol Phys 2017;99:876-83. [Crossref] [PubMed]
- Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 2011;117:4060-9. [Crossref] [PubMed]
- Scorsetti M, Comito T, Tozzi A, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol 2015;141:543-53. [Crossref] [PubMed]
- Agolli L, Bracci S, Nicosia L, et al. Lung Metastases Treated With Stereotactic Ablative Radiation Therapy in Oligometastatic Colorectal Cancer Patients: Outcomes and Prognostic Factors After Long-Term Follow-Up. Clin Colorectal Cancer 2017;16:58-64. [Crossref] [PubMed]
- Pasqualetti F, Montrone S, Vivaldi C, et al. Stereotactic Body Radiotherapy in Patients with Lung Oligometastases from Colorectal Cancer. Anticancer Res 2017;37:315-9. [Crossref] [PubMed]
- Ibrahim T, Tselikas L, Yazbeck C, et al. Systemic Versus LocalTherapies for Colorectal Cancer Pulmonary Metastasis: What to Choose and When? J Gastrointest Cancer 2016;47:223-31. [Crossref] [PubMed]
- Carvajal C, Navarro-Martin A, Cacicedo J, et al. Stereotactic body radiotherapy for colorectal lung oligometastases: preliminary single-institution results. J BUON 2015;20:158-65. [PubMed]
- Takeda A, Sanuki N, Kunieda E. Role of stereotactic body radiotherapy for oligometastasis from colorectal cancer. World J Gastroenterol 2014;20:4220-9. [Crossref] [PubMed]
- Wild AT, Yamada Y. Treatment Options in Oligometastatic Disease: Stereotactic Body Radiation Therapy - Focus on Colorectal Cancer. Visc Med 2017;33:54-61. [Crossref] [PubMed]


