Medicine adaptive pathways to patients (MAPPs): using regulatory innovation to defeat Eroom’s law
Introduction
Moore’s law is an axiom for innovation and productivity. Attributed to Gordon Moore, Fairchild Semiconductor’s Director of research & development (R&D) in 1965 (1), Moore predicted that microprocessing power and speed would double every 2-year period. His ‘law’, self-fulfilling or not, remains unbroken as the cost of computing power continues to decline while speeds get faster at a lower cost to the consumer, doubling in productivity roughly every 24 months.
Unfortunately, Moore’s law has a doppelganger—an evil twin, who inhabits the land of pharmaceutical research. Eroom’s law first appeared in a now infamous Nature article in March of 2012, and was coined by industry analyst Jack W. Scannell (2). It is, literally, Moore’s law in reverse.
Eroom’s law finds that the pharmaceutical sector invests $50 billion annually in research for new medicines (3), but “the number of new drugs approved per billion US dollars spent has halved roughly every 9 years since 1950, falling around 80-fold in inflation-adjusted terms” (2). While many assume this decline in pharmaceutical productivity is simply a matter of management oversight or poor performance, a more rational investigation finds that the causes are considerably more complex, and the solutions even more challenging.
The 2013 Priority Medicines Report for Europe by the World Health Organization (WHO) outlines with great precision the largest areas of unmet medical need and disease burden in Europe. The top three treatment areas where new therapies are required are cardiovascular disease, psychiatric disorders such as Alzheimer’s disease, and oncology (4). Unfortunately, research presented at Pharma CI 2012 (Figure 1) highlights the challenges faced by many pharmaceutical companies when trying to develop new medicines in these three vital areas (5).
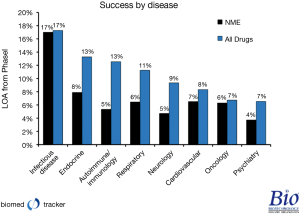
Pharmaceutical companies have invested enormous sums of time, effort and energy in new molecular entities (NME) in the areas of unmet medical need identified by the WHO, but the approval rates from phase I are 7% for cardiovascular disease, dropping to 4% for Alzheimer’s disease. In fact, Alzheimer’s research has been spectacularly challenging as, from 1998 through 2011, drug developers have only had three therapies approved while 101 NMEs were abandoned, many of those after phase III trials were conducted (6).
When looking at Eroom’s law in the context of cardiovascular disease, Alzheimer’s disease, and cancer, the increasing cost of R&D is not only a factor of research management quality, but also indicative of an industry trying to address therapeutic areas that have incredibly complex biological mechanisms with budget-crushing failure rates. In order to move science forward and meet these daunting medical challenges for patients, new collaborative approaches to testing the efficacy and effectiveness of new improved medicines should be embraced by regulators in close partnership with patients, payers, and practitioners. To not do so puts the entire healthcare value chain, and ultimately the wellbeing of patients, at risk.
MAPPs: a new regulatory paradigm
Medicine adaptive pathways to patients (MAPPs) build on the stratification breakthroughs of personalized medicine to facilitate new types of clinical trials that adapt to a given patient’s response. One of the earliest examples of an adaptive trial was launched in 2008 by Dr. Don Berry, head of Quantitative Sciences at M.D. Anderson Cancer Center in Houston Texas for breast cancer, under the moniker of I-Spy 2 (7).
The key innovations of the I-Spy 2 trial are the ability to use biomarkers to pre-identify those patients who will more likely respond to therapy, cut the required number of patients of a breast cancer phase III drug trial by a full logarithm of power from 3,000 to 300 participants, and increase the likelihood of having a successful trial from 30% to 85%. As of December 3, 2013, I-Spy 2 has successfully cleared its trial endpoints, and is moving on to I-Spy 3 (8).
Thinking of a MAPPs trial visually (9), it will launch at the centre of a ball in phase I/II with a well-defined population that is likely to respond based on available diagnostics for therapies with a high level of efficacy and safety (Figure 2). Where the proposed MAPPs pathway in Europe diverges from I-Spy is the desire, once phase II is completed and mechanisms and endpoints verified, to move forward seeking confirmatory evidence with a limited market authorization for sale while phase I/II trials continue for other indications. In this way, MAPPs will provide a limited commercial marketing authorization for a patient group who has access to new therapeutic agents while validating additional clinical endpoints at the same time. This means that MAPPs could have the theoretical ability to run trials that fulfil both the efficacy requirements for authorization and the effectiveness needs of national health technology assessments (HTA) simultaneously, while also providing patients with needed therapies in the most efficient timescale and trial size possible.
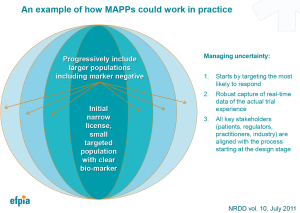
As more indications are validated, the population will theoretically grow with the expanding evidence base. A key to this limited authorization will be the harnessing of ‘real world evidence’ to monitor the approved cohort with new tools and strategies being developed and tested under the Digital Agenda mandate of the European Commission (10).
While this construct may seem somewhat revolutionary, there is continuing evidence gathering and research in this methodology. The NewDigs platform at Massachusetts Institute of Technology (MIT) is a thought leader in investigating new regulatory pathways, and has been consulting to the MAPPs taskforce in its formulation and structure. Findings of NewDigs have the endorsement and participation of Hans Georg Eichler, Senior Medical Officer of the European Medicines Agency (EMA), and a recently published study states quite clearly, “full authorization is in name only, because continued surveillance may reveal rare adverse events or other information that could lead to further adjustments to the drug label and/or treated population. A true (adaptive licensing) scheme is continuous throughout the life span of the drug, although data generation needed for later-stage authorizations/modifications may be based largely on analysis of observational data or on (randomized clinical trials) for use in other therapeutic settings.” (11).
The initial investigations and feasibility studies of MAPPs in the European Union (EU) will be under the auspices of the innovative medicines’ initiative (IMI), one of the world’s largest public private partnership research programs. The next phase under IMI2 is launching in 2014 with a budget in excess of €3.25 bil, €1.5 bil being contributed by the European Federation of Pharmaceutical Industries and Associations (EFPIA) (12).
The regulatory research objectives of MAPPs will be placed into an IMI2 proposal pipeline for validation. They will be formulated and agreed upon by the IMI2 ‘Think Tank’, including stakeholders from patient groups, regulators, research, industry, and medical practice. The Think Tank will be chaired by the research group CASMI, a joint partnership of University College London and Oxford University, supported by the Wellcome Trust (13).
MAPPs in Europe: challenges and opportunities
While there is mounting evidence that adaptive pathways can help move needed therapies to patients safely and efficiently, the European regulatory environment provides its own unique challenges to MAPPs. The approval of successful trials for new therapies rests with the Committee for Medicinal Products for Human Use (CHMP) of the EMA and national competent authorities, but the European Commission’s Directorate General for Health and Consumers (DG SANCO) has oversight over the CHMP/EMA’s decisions. A MAPPs pathway will use the current best practice in regulatory sciences, seeking a commercial authorization based on evidence gained in phase II. However, there have recently been instances where such approaches have been rejected by DG SANCO, overturning positive rulings of the CHMP/EMA that used evidence bases outside the clinical trial structure, including 20 years of positive surrogate data (14,15).
There is a belief amongst many regulators that randomized controlled trials (RCTs), once they have validated phase III endpoints, remove any doubts around efficacy, safety, and performance of new experimental therapies. While RCTs are a general barometer of a new drug’s potential future performance, the continuing movement of new therapies into secondary indications and disease areas with complex genetic mutations make them less valuable when seeking to stratify a population.
Future clinical trial designs should provide valuable knowledge and information as to which patients respond to a particular therapy and why. A recent editorial in the New York Times highlights the problems with RCTs when considering the opportunities provided by innovation in stratified medicines, “more than 600 brain cancer patients were randomly assigned to two evenly balanced groups..what’s more, the study was “double-blind”—neither the patients nor the doctors knew who was in which group until after the results had been assessed. The centerpiece of the country’s drug-testing system—the randomized, controlled trial—had worked, except in one respect: doctors had no more clarity after the trial about how to treat brain cancer patients than they had before. Some patients did do better on the drug… (but) the trial was unable to discover these “responders” along the way, much less examine what might have accounted for the difference.” (16).
Another problem with the current approach to RCTs is that the participating population is often younger than those who will receive the drug in real world practice. This age difference can be reflected in a lack of co-morbidities in the RCT compared to what is seen when treating those over 65 years of age (16). This situation is often a byproduct of RCTs in general, as the exclusion criteria are selected by sponsors to enable testing a population where the true efficacy and safety of the specific therapy can best be demonstrated (17).
MAPPs are a move away from the traditional binary ‘on/off’ approach to the approval of new medicines as practiced in the RCT. The regulatory systems around this new approach must be aligned, and regulators must accept that a RCT is not a guarantee of (I) statistical certainty; (II) a predictable safety profile; or (III) the actual performance of a new therapy in the real world.
MAPPs gain valuable information about multiple endpoints while making a new therapy available to patients in as timely a fashion possible when supported with data for a targeted indication. This approach should also incur fewer adverse events and less toxicity, as the limited initial license will be focused on those most likely to respond. It can also radically reduce the time to market for new therapies, relieving pressure on the exponentially increasing costs of RCTs.
While I-Spy 2 has shown great uptake for trial participation by those with aggressive breast cancers, a better understanding of the willingness of patients, payers and regulators to operate in areas of increased uncertainty will need to be investigated under the auspices of IMI2. Uncertainty does not equate to increased risk, and the recent experience of companies in Europe trying to move beyond the current rigid clinical trial system outlines the lack of alignment at the regulatory level on the interpretation of acceptable benefit risk when weighed against patient needs at the regulatory level. Agreement on these key parameters must be achieved as part of the scientific advice with EU regulators when implementing MAPPs.
In addition, all key stakeholders must be aligned and agree on the evidence package for early approval and re-assessments at the design phase of MAPPs, which is a particular challenge in Europe. Not only does a new therapy need to have the approval of both EMA and an increasingly active DG SANCO, but it also requires ‘buy-in’ from the 28 different member states and multiple HTA authorities who ultimately decide to pay for new therapies. Each member state has its own distinct health priorities, and reimbursement for new therapies is a sovereign choice of each national health authority, not of Brussels. If the 28 member states in Europe do not accept the value of MAPPs, there will be no way to ultimately pay for new medicines that are licensed by EMA to enter the marketplace.
Further, a key component of MAPPs will be need to monitor in real-time the response of patients to new therapies, both for better outcomes, and avoidance of adverse events. The information technology (IT) infrastructure and interoperability to provide the needed evidence base across multiple regional and national health jurisdictions is currently lacking in the EU, but there are programs running to investigate and support enhanced health informatics and electronic patient records. These will need to be tested, proven, and implemented in conjunction with MAPPs platforms under IMI2.
Finally, as the evidence base for a new therapy grows and evolves, pricing and reimbursement must be able to respond to market demands. There must be willingness by national regulators to address a flexible pricing structure that responds both upwards and downwards based on the evolution of data and knowledge gained in the course of a MAPPs development plan.
MAPPs: getting needed medicines to patients
Ultimately, MAPPs supports the patient’s need for timely access to effective innovative medicines. It will reduce research barriers and improve efficacy, increasing innovation in the life sciences creating benefits to society. It can improve the efficiency of health care delivery, providing the data required to evaluate new therapies in a more timely fashion while supporting innovation, increasing effectiveness, and promoting investment by the European pharmaceutical sector.
Eroom’s law is a manifestation of the mounting challenges to the pharmaceutical industry due primarily, and ironically, to their past successes. The pharmaceutical industry’s blockbuster discoveries from 20 years ago are now generic medicines, and the next generation therapies lay at the edges of scientific knowledge, targeting infinitely more complex diseases. MAPPs can help address these challenges, providing the platform for improved outcomes for patients while ushering in a much more efficient system for all stakeholders in Europe. While Eroom may be a law today, ultimately, some laws are made to be broken.
Acknowledgements
Disclosure: Duane Schulthess is a paid consultant to pharmaceutical companies and owns shares in Shire PLC; Magda Chlebus receives a salary to represent the interests of pharmaceutical companies; Richard Bergström receives a salary to represent the interests of pharmaceutical companies; Karin van Baelen is an employee of Johnson & Johnson.
References
- 1965 – “Moore’s Law” Predicts the Future of Integrated Circuits. Computer History Museum, Mountain View, CA. Available online: http://www.computerhistory.org/semiconductor/timeline/1965-Moore.html
- Scannell JW, Blanckley A, Boldon H, et al. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 2012;11:191-200. [PubMed]
- Kaitin KI. The Landscape for Pharmaceutical Innovation: Drivers of Cost-Effective Clinical Research. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3150117/
- Kaplan W, Wirtz VJ, Mantel-Teeuwisse A, et al. Priority Medicines Report for Europe and the World 2013 Update. WHO (Internet) 2013;57. Available online: http://www.who.int/medicines/areas/priority_medicines/MasterDocJune28_FINAL_Web.pdf
- Hay M, Thomas DW, Rosenthan J, et al. BioMedTracker Clinical Development Success Rates for Investgational Drugs. Pharma CI 2012;15. Available online: http://www.pharmaciconference.com/files/Clinical_Development_Success_Rates_for_Investigational_Drugs.pdf
- McBride R. Pharma counts just 3 Alzheimer’s drug wins in 13 years (101 losses!). Available online: http://www.fiercebiotech.com/story/pharma-counts-just-3-alzheimers-drug-wins-13-years-101-losses/2012-09-14
- Winslow R. A New Rx for Medicine. The Wall Street Journal 2010. Available online: http://online.wsj.com/news/articles/SB10001424052748703882404575520190576846812
- Puma Biotechnology Reports Positive Top Line Data from I-SPY 2 TRIAL. The Wall Street Journal 2013. Available online: http://online.wsj.com/article/PR-CO-20131204-910607.html
- Eichler HG, Abadie E, Breckenridge A, et al. Bridging the efficacy–effectiveness gap: a regulator’s perspective on addressing variability of drug response. Nature Reviews Drug Discovery 2011;10:495-506. Available online: http://www.nature.com/nrd/journal/v10/n7/abs/nrd3501.html?message=remove&lang=en?WT.ec_id=NRD-201107 [PubMed]
- Digital Agenda for Europe. eHealth studies: an overview. Available online: https://ec.europa.eu/digital-agenda/en/news/ehealth-studies-overview
- Eichler HG, Oye K, Baird LG, et al. Adaptive Licensing: Taking the Next Step in the Evolution of Drug Approval. Clinical Pharmacology & Therapeutics 2012;91:426-37. [PubMed]
- IMI2 2013. Available online: http://www.imi.europa.eu/content/imi-2#Budget
- Centre for the Advancement of Sustainable Medical Innovation 2012. Available online: http://casmi.org.uk/
- Mahony H. Life-saving drug hits eurocrat wall - a Kafkaesque tale. EU Observer 2013. Available online: http://euobserver.com/social/119040
- European Parliament, Parliamentary questions 2011. Available online: http://www.europarl.europa.eu/sides/getAllAnswers.do?reference=P-2011-009469&language=EN
- Leaf C. Do Clinical Trials Work? The New York Times 2013. Available online: http://www.nytimes.com/2013/07/14/opinion/sunday/do-clinical-trials-work.html?_r=0
- White Paper: U.S. Food and Drug Administration (FDA) Inventory of Clinical Trials Protocols and Clinical Study Data 2011. Available online: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/ConductingClinicalTrials/UCM309552.pdf


