The changing world of drug development: an academic research organization’s perspective on the “Seven Wonders” of the future world of anticancer drug development
Introduction
“There is no treatment”, this is the oldest known description of cancer, written on Egyptian papyrus around 3000BC, the era in which the Egyptians built the great pyramid of Giza, the oldest of the Seven Wonders of the ancient world. Through human history, the fight against cancer has never ceased. Enormous progress was achieved after the 19th century with use of the modern microscope in studying disease tissues (1). Over the past decades, survival rates for many cancers have increased impressively by the introduction of prevention, new drugs and multi-disciplinary treatments. In breast cancer, the five-year relative survival rate has soared from 63% in the early 1960s to 90% (2). Cancer no longer means “incurable disease”, and can be controlled in many patients. The World Health Organization (WHO) lists cancer as a chronic disease and in parallel with an aging population, the number of cancer patients is increasing. According to the latest world cancer statistics (GLOBOCAN 2012) (3), an estimated 14.1 million new cancer cases occurred in 2012, compared with 12.7 million in 2008; by 2025, there will be nearly 20 million new cancer cases per year. Cancers cost the European Union 124 billion Euros each year (4), about 10% of the total expenditure on healthcare. With limited resources, healthcare payers will in the future likely only pay for “performance” rather than for service. Treatments that prevent diseases, cure life-threatening diseases, reduce the overall use of resources, and let patients stay as productive as possible: these are the approaches which the payers will support.
Drug developers are facing enormous challenges, including the productivity crisis of their own. Between 2002 and 2011, the pharmaceutical and biotech sector spent nearly 1.1 trillion dollars on research & development (R&D), and the United States Food and Drug Administration (FDA) approved 308 new molecular entities and biologics in the 10 years to 2011. That means the average cost per approved molecule ranged from 2.3 to 4.9 billion dollars (5). Only about two of every ten marketed drugs generate sufficient revues to cover their associated R&D cost (6). Compared to other clinical medicine, oncology has the highest attrition rate for late stage clinical trials, and overall success rate from first-in-man to approval is about 5% (7).
Cancer covers a complex and heterogeneous area of diseases and no two tumors, even of the same origin and histology, are identical. The previous perception of cancer as a distinct organ-specific disease is replaced by one of smaller entities responding to different biological pathways. Medical science is dynamic and making rapid progress, with massive investments in life sciences and availability of performing information technology (IT) tools. In 2001, to sequence an entire human genome cost 95 million US dollars. Today, it is done in a matter of hours at a cost of only 1,000 dollars. Pharmacogenomics helps us to understand how genetic variation affects individual response to therapy, with the twin aims of optimizing drug therapy and ensuring maximum efficacy with minimal side effects. Inexpensive and rapid gene sequencing may change the future medical practice. Yesterday’s challenge is today’s practice of a relatively large armamentarium of anti-cancer weapons; today’s challenge will be tomorrow’s practice of optimizing the use of treatments and translation of biology into therapeutic decisions. We have never had so many interactions between bench and bedside. Will those advanced techniques create the wonders of a new anti-cancer world?
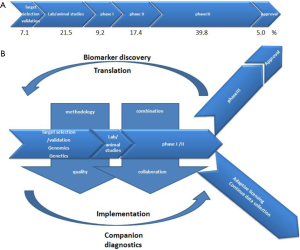
Cancer drug developers need to build on our assets, and we propose to build on what could be depicted as “the seven wonders” for the future anticancer medicine world (Figure 1). The art will be learning how to articulate and combine them together.
Biomarkers
It is well recognized that patient’s genetic make-up and the tumor’s molecular profile can influence an individual’s response to specific therapies. A new era of personalized medicine has dawned in which therapy should be tailored to an individual’s disease and genetic profile. Effective integration of biomarkers into clinical drug development programs has been identified as a key prospect in the FDA’s Critical Path document (8). In novel drug development, the predictive value of validated biomarkers could inform crucial go/no go decisions around safety and efficacy. Biomarkers require the development of companion diagnostic tests able to select patients who are likely to respond to a given molecule, a “preselected” or “enriched” patient population. For example, panitumumab is a human monoclonal antibody to epidermal growth factor receptor (EGFR), approved for the treatment of patients with metastatic colorectal cancer, however the efficacy of panitumumab was restricted to patients with wild-type KRAS genes, while patients with mutated KRAS did not benefit. In addition, biomarkers are also used as a method to determine therapeutic interventions. It is challenging to develop an exact dose intervention that fits all patients due to the inter-individual variability. It may be possible to have a dose adjustment based on the genetic polymorphisms of drug-metabolizing enzymes or drug transporter proteins. Some drug labels have already been updated with biomarker information. For example, the azathioprine label added information related to thiopurine methyltransferase, and recommends that patients with thiopurine methyltransferase deficiency or lower activity are at increased risk for myelotoxicity. A list of valid genomic biomarkers identified in approved drug label could be found on the FDA website (9), which better informs healthcare professionals, and thereby helps to provide better patient care.
Effective integration of biomarkers into drug development may facilitate and accelerate drug approval and promote personalized medicine. Nevertheless, the biomarker implementation into clinical practice or used in drug development must be pragmatic and carefully evaluated. As said by Artemas Ward, a famous American journalist: “It is not so much the things we don’t know that get us in trouble. It is the things we know that aren’t so”. Overpromising the utility of new biomarkers without adequate evidence will only hamper its usefulness in drug development and clinical therapy. The FDA issued a “Guidance for Industry: Pharmacogenomics Data Submissions” in 2005 (10), with the purpose of creating early interaction between regulatory and stakeholders without making a regulatory decision, allowing biomarker-driven drug development to move forward effectively.
Imaging
Imaging is another medical technique that benefits from the computer science revolution. Non-invasive imaging methods are widely used in cancer research for staging, diagnosis, response assessment, monitoring, etc. In drug development scenarios, tumor response is conventionally assessed by measuring the percentage of reduction in tumor size after chemotherapy. Vigorous debate has challenged the use of anatomic assessments alone, as it may take two or three months to detect any shrinkage. Some molecular imaging techniques provide an insight to tumor microenvironment in vivo and predict response or non-response at very early stage. For example, early metabolic response evaluation by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) allows for an accurate prediction of histopathologic response during neoadjuvant chemotherapy in esophago-gastric cancer (11-13). The most important finding of these previous studies is the fact, that for patients not achieving a decrease of the FDG-uptake of ≥35% at 2 weeks after the start of chemotherapy, the probability of responding after multiple doses is slim (FDG-PET negative predictive value is more than 95%). A reliable early identification of non-responding patients will be extremely valuable in guiding management and treatment, and to avoid that non-responsive patients are receiving unnecessary toxicity related to therapy. These advanced imaging technologies will allow for earlier selection of candidates for new drugs, and thereby reducing the large attrition rate in the pharmaceutical development process. Ultimately, patients will more rapidly have access to better treatments.
New imaging biomarkers are constantly being developed, and need to be qualified for full use in clinical research. Drug developers need to feel confident that a measured change in the imaging biomarker faithfully reflects the desired change in the underlying tumor pathology. Although imaging technologies such PET and magnetic resonance imaging (MRI) have existed for decades and are widely available, their full use in clinical research remains challenging. The quality and the comparability of images collected within international multi-center clinical trials are not optimal. Clinical research involving imaging can only be achieved within robust, quality assured, multi-site clinical trials supported by robust methodology and operational infrastructures allowing the processing, storage, and analysis of imaging data, which should be fully integrated with clinical and biological data.
Quality assurance
Access to good quality biosamples or imaging data for translational research is fundamental to personalized cancer treatment; however, there are often major bottlenecks. For one thing, new skills are required to build on new platforms to integrate clinical, biological, and imaging data in the decision making process to control attrition rate of new drugs and/or decide on molecular sub-entities that will ultimately benefit new therapeutic strategies. In addition, currently there is no clear worldwide consensus on the criteria or level of quality assurance required either for different types of biomarker assay or for functional/molecular imaging when those novel diagnostics are implemented in multi-center clinical trials.
An infrastructure should implement common principles and guidelines for appropriate levels of quality assurance for biosample collection (e.g., centralized biobanking) with a sample tracking system, appropriate assay methods, and accredited laboratories to perform biomarker testing, supporting data management with biostatistics and bioinformatics experts for molecular data analysis and reporting. Some screening platforms are now emerging in clinical trials, as an example serves here the European Organisation for Research and Treatment of Cancer (EORTC) Screening Patients for Efficient Clinical Trial Access (SPECTA). The SPECTA colorectal cancer platform (SPECTAcolor) is the first prospective tumor tissue biobank and centralized biomarker analysis infrastructure for genetic profiling aiming at easy and targeted clinical trial access. Biosamples are collected with standard operational procedures, centrally tracked by the EORTC Headquarters using modern web-based IT tools. Systematic analysis and documentation enables us to establish reliable databases of patient molecular information, so as to enroll patients into prospective biomarker-driven clinical trials.
Implementing functional imaging as companion diagnostics to assess response requires that an observed change of the imaging biomarker due to treatments must be greater than the intrinsic and extrinsic variability of the biomarker in the absence of treatment. High reproducibility of molecular and functional imaging techniques relies on good quality data and standardized procedure. In response to the challenges of imaging biomarker qualification, the Quantitative Imaging in Oncology: Connecting Cellular Processes to Therapy (QuIC-ConCePT) consortium was created and resourced by the Innovative Medicines Initiative (IMI), Europe’s largest public-private initiative. It aims to qualify three specific imaging biomarkers of tumor cell proliferation, apoptosis, and necrosis, to allow drug developers to demonstrate reliably the modulation of these pathologic processes in tumors of patients in future trials. The qualified imaging biomarkers will help drug developers in decision-making during phase I trials of investigational therapies, confident that the biomarkers are robust, consistent in multiple cancer centers, and reflective of the desired change in the underling tumor pathology.
Clinical trial methodology
Drug development requires methodologically robust and practice-relevant clinical trials. Optimized phase II trial design with reasonable endpoints based on a strong biological rationale, will help to facilitate smaller, faster, and less expensive confirmatory phase III trials, or even give enough data to draw the necessary conclusion after phase II study and lead to successful drug approval. There are examples of blockbuster drugs that were approved by the FDA without phase III results, thanks to their vastly improved activity and limited toxicity relative to the standard of care for the disease under investigation. Imatinib (Glivec®, Gleevec®, Novartis), was developed rationally targeting bcr-abl protein. This protein is over-expressed in chronic myeloid leukemia (CML) due to a pathognomonic and driving fusion gene, leading to interrupted production of white blood cells in patients. The results of imatinib’s preliminary studies were dramatic: nearly every CML patients responded and only minimal side effects were reported. Before imatinib, prognosis was dismal and only 3 out of 10 patients survived 5 years, today the 5-year survival rates are up to 90%. The first phase I study began in June 1998, and the drug received the FDA approval in May 2001, only 10 weeks after the new drug application was submitted (14). Unfortunately, success stories like this are rare. We need more so-called “rational drug designs” in the future.
A study will have a large probability of failure if it is based on inaccurate estimations or unrealistic assumptions. Changes in design or analyses guided by the accumulated data at an interim point in the trial may make studies more efficient. For example, a conventional design to find optimal dose-response might use multiple fixed-size randomized groups to ensure that an optimal dose level is included, but including several groups with sub-optimal doses will decrease the study efficiency. An adaptive design can ascertain when further data collection for a particular group is not useful, and stop data collection, decreasing the cost and time while, when planned upfront and adequately, allows keeping the study’s integrity. Similarly, an adaptive design approach that can adjust the study sample size to avoid underpowered studies, e.g., the initial assumption of variance is too low or of treatment-effect size is too large.
Smart but robust clinical research methodology using adaptive design could shorten the trial duration, the size of the studied population and ultimately the trial costs. Drug developers should invest more money early on the knowledge of the molecular basis of a disease to reduce the risk of losing a lot of money and time further down the line. Modern clinical trial methodologies propose a wide range of adaptive designs that should be selected according to the research question e.g., phase II adaptive design, or “pick the winner”.
Combined treatment
Combination therapy whether it is combination of drugs, inhibition of multiple pathways or combination of modalities (e.g., combined chemo-radiotherapy) is of great importance in many disease settings, and in particular in oncology. Advances in biology and genomics have led to more development of targeted agents. However, modulation of one target may not be sufficient enough to achieve durable response. The use of multiple therapeutic agents is more likely to lead to therapeutic advances, as tumors may be driven by several pathways. New therapeutic approaches using combinations of drugs (multiple therapeutic targeted agents, or targeted agents + chemotherapy) can help to improve treatment response, minimize development of resistance, or minimize adverse events. Therefore, it is expected that co-development of two or more new combination drugs will increase.
Combinations of two or more new therapeutic entities may be required to achieve measurable clinical benefit. However, parallel co-development in combination of two new agents has its own challenges, as it will generally not provide sufficient information about safety and effectiveness of each of the individual new investigational drug. Ethical concerns and low efficacy when used alone may prevent conducting parallel-group comparison with each individual compound. On the other hand, for pharmaceutical companies, the combination of new compounds in the early development increases the risk of failure, since knowledge about drug interactions may be missing. Another obstacle for combining different drugs is the fact that the involvement of several companies within one trial creates challenges to data sharing, intellectual property and marketing concerns. The FDA has recently provided guidelines on the standard for licensing of new-new combination (15). According to these guidelines, the combination should be based on a strong biological rationale and promising preclinical data, proving that the combination is superior to existing treatments or to the individual agents alone. An early interaction with the FDA is suggested on the appropriateness of co-development before initiation of clinical development of a combination.
Adaptive licensing (AL)
Traditional drug licensing is a binary decision, divided into two distinct phases: pre-licensing and post-licensing. During the pre-licensing phase, patients can only be exposed to a new drug/investigational product in clinical trials with informed consent procedures and specific eligibility criteria. Once approved, the drug is prescribed in the real world populations who need not to meet specific eligibility requirements. It is unrealistic to expect matching safety and effectiveness when a drug is used in a heterogeneous real-world population based on limited trial data. AL is based on stepwise learning, progressive management and reduction of uncertainty. It allows earlier access to patients with unmet medication needs (e.g., life-threatening disease), and patients, doctors, and regulators are all willing to take greater risks, including unknown efficacy and safety. Thus, the evaluation of AL is not binary but a continuum; pre- vs. post-licensing stages will be replaced by graded, more timely and cost-effective market entry, leading to greater market stability.
The idea of staggered approval was introduced in the European Medicines Agency’s (EMA) Road Map to 2015 as a possible solution for earlier approval of drugs to reduce barriers to innovation, and provide timely access for patient with unmet medication needs. Similar proposals for adaptive approaches to drug licensing have emerged worldwide under various labels, including progressive licensing (Canada), accelerated approval (US), adaptive approval (Singapore) (16). These proposals vary in details, but all are based on continuous evaluation over time. Access to new drugs will combine data from clinical trials, observational data and effectiveness of drugs in real-world use and access control.
AL may in some cases reduce time to full market approval. Although it should not be expected to reduce overall attrition rate, AL may reduce the overall cost of development by reducing expensive late stage failure and post-market withdrawals. Is it a new wonder for the anticancer medicine world? “Not a panacea, but necessarily a route for all drugs, one size doesn’t fit all”, are comments received from regulatory bodies (17). The AL pathway to market will likely vary on a case-by case basis. For a product used to treat a life-threatening disease, the quantity of data required for an initial authorization might be considerably less than that for a product used to treat a disease for which many treatments are available. The AL pathway for any drug should be based on the willingness of patients, doctors, payers, and regulators to accept a great level of uncertainty in term of safety and efficacy. Another important point is that all stakeholders will need to accept that initial approval is not just early but also conditional. Hence, a clear commitment is required from developers to continue conducting studies to collect data after initial licensing. “What is the appropriate regulatory action to take in the event that promised studies are not performed or expected data do not become available” is a critical issue as has been prominently highlighted by Avorn in 2007 (18). Taking no action would undermine the system, whereas restrictions of the license due only to lack of new data will, in the case of a potential lifesaving drug, be difficult to accept for patients and doctors. A wonder may become a mirage, a cruel illusion. Therefore, proper management and communication might be the best or only option to balance the regulators’ gatekeeper and enabler roles. Harmonization of the development plan across drug developers, regulators, and payers could be the way to ensure that sponsors continue to monitor their products and to collect data on the effectiveness of drugs in use.
New forms of multi-stakeholder collaboration
Pharmaceutical companies are collaborating in co-development of their individual products, offering a double-team approach to fight cancer. It started in 2009, when AstraZeneca and Merck formed a partnership to evaluate combination candidates and share all development costs. One of their success stories involves their capitalizing on the synergies of two different drug mechanisms, a MEK inhibitor (AZD6244) and a protein kinase B inhibitor (MK-22060) in non-small cell lung cancer, and speeded the development time (19). Similarly, Bristol-Myers Squibb and Roche are collaborating on a melanoma product (vemurafenib). In September 2012, ten leading biopharmaceutical companies formed a non-profit organization, TransCelerate BioPharma, with the mission to share research and solutions that will simplify and accelerate the delivery of exciting new medicines for patients.
A new era of drug development will require strong collaboration between the industry and academics, as well as close communication with regulators and payers (20). Academic research organizations can provide scientific advice and clinical trial methodology, and also incorporate additional translational research projects. Commercial research organizations can support site monitoring and operational management. Industry can provide new agents as well as input for regulatory aspects. The responsibility split could maximize the strengths of each stakeholder.
Conclusions
Thanks to high technology, drug developers’ future has never been so promising. We described in this review how clinical research and drug development may evolve in the coming years. The molecular determinants of cancer will be more known and better translated into new drugs and companion diagnostics. However, on average, pharmaceutical companies spend only 7% of their budget on target/mechanism selection and validation (5) (Figure 2A). We suggest adjusted investment percentages in future drug development (Figure 2B), with pharmaceutical companies increasing investment of its R&D budget in target selection and validation based on genomic and genetic information; animal models should, by contrast, be used much less frequently because they provide an inaccurate means of predicting efficacy in humans. Optimized phase I/II studies based on a strong biological rationale will help to have more efficient and smaller phase III trials and lead to successful drug approval. AL could be another regulatory approach allowing patients with life-threatening diseases to access novel treatments. Having good biological, imaging and clinical data for translational research is fundamental to personalized medicine, and eventually those achievements in bench side will bring high benefits to bedside when companion diagnostics are implemented in clinical research. Conducting modern clinical cancer trials demands optimized clinical methodology, good quality data, combined therapy, and multi-stakeholder collaboration, so as to accelerate patient access to new treatment and techniques.
Acknowledgements
The authors like to thank the support of Fonds Cancer (FOCA) from Belgium.
Disclosure: The authors declare no conflict of interest.
References
- The History of Cancer. Available online: http://www.cancer.org/acs/groups/cid/documents/webcontent/002048-pdf.pdf
- AACR Cancer Progress Report 2012. Available online: https://www.aapm.org/meetings/documents/2012_AACR_CPR.pdf
- Latest world cancer statistics. Available online: http://www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf
- Economic burden of cancer in Europe exceeds 124 billion euros a year. Available online: http://www.thepharmaletter.com/article/economic-burden-of-cancer-in-europe-exceeds-124-billion-euros-a-year
- Pharma 2020- From vision to decision. Available online: http://www.pwc.com/gx/en/pharma-life-sciences/pharma2020/vision-to-decision.jhtml
- Pharmaceutical Research and Manufacturers of America (PhRMA) 2011 profile. Available online: http://www.phrma-jp.org/archives/pdf/profile/PhRMA%20Profile%202011%20FINAL.pdf
- Bria E, Di Maio M, Carlini P, et al. Targeting targeted agents: open issues for clinical trial design. J Exp Clin Cancer Res 2009;28:66. [PubMed]
- Innovation or Stagnation? Critical Path Opportunities Report. Available online: http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/UCM077254.pdf
- Table of Pharmacogenomic Biomarkers in Drug Labeling. Available online: http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm
- Guidance for Industry Pharmacogenomic Data Submissions. Available online: http://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126957.pdf
- Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 2003;21:4604-10. [PubMed]
- Ott K, Herrmann K, Krause BJ, et al. The Value of PET Imaging in Patients with Localized Gastroesophageal Cancer. Gastrointest Cancer Res 2008;2:287-94. [PubMed]
- Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 2007;8:797-805. [PubMed]
- The Story of Gleevec. Available online: http://www.innovation.org/index.cfm/StoriesofInnovation/InnovatorStories/The_Story_of_Gleevec
- Guidance for Industry Codevelopment of Two or More New Investigational Drugs for Use in Combination. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM236669.pdf
- Eichler HG, Oye K, Baird LG, et al. Adaptive licensing: taking the next step in the evolution of drug approval. Clin Pharmacol Ther 2012;91:426-37. [PubMed]
- Adaptive Licensing: a useful approach for drug licensing in the EU? Available online: http://www.topra.org/sites/default/files/assets/pdf/1___session_4_-_adaptive_licensing_-_ema_hans_georg-eichler_29_nov_2012.pdf
- Avorn J. Paying for drug approvals—who’s using whom? N Engl J Med 2007;356:1697-700. [PubMed]
- Big Pharma + Big Pharma = Collaborative Approach to Fight Cancer. Available online: http://www.citeline.com/thought-leader-blog/big-pharma-big-pharma-collaborative-approach-to-fight-cancer/#sthash.5E2fOZUs.dpuf
- Lacombe D, Burock S, Meunier F. Academia-industry partnerships: are we ready for new models of partnership?: the point of view of the EORTC, an academic clinical cancer research organisation. Eur J Cancer 2013;49:1-7. [PubMed]



