Organizing a multidisciplinary clinic
Introduction
Coordinating the management of patients with cancer is uniquely challenging. Few other diseases rely so heavily on the integration and expertise of multiple medical specialists, including those from medical oncology, radiation oncology, pathology, radiology, surgery, and pain specialists. Furthermore, supportive care is crucial and can be difficult to coordinate—patients often need extensive management by social work, psychosocial oncology, nutrition, and other services. The last few decades have seen numerous dramatic advancements in oncology, both in our understanding of the pathophysiology of malignancies and in the development of specific treatment paradigms. In order to realize the benefits of these advances and maximize optimal care delivery, multidisciplinary care has emerged as an ideal therapeutic platform for patients with cancer. Two of the most widely accepted forums for this comprehensive care approach have been multidisciplinary tumor boards (MDTB) and multidisciplinary clinics (1,2).
Multidisciplinary tumor boards (MDTB)
The tumor board is a long-established means of bringing together clinicians from different fields to discuss the optimal management of individual cases (3). Most MDTB usually occur weekly or monthly, with representatives from all fields of oncology meeting in a combined forum. Two fundamental goals are accomplished with multidisciplinary care: clinician education and improved treatment planning. At the clinician level, expertise from each discipline is shared in an open didactic environment. In doing so, perspectives and recent advances in a particular field are shared by clinicians practicing in other arenas, thereby promoting a wider knowledge base for future care. Concurrently, at the patient care level, the discussion generated in an individual case optimally leads to a consensus treatment recommendation; in turn, the combined recommendation reassures the patient that his or her care plan is supported by sound evidence and broad clinical support. The treatment plan generated at a MDTB is also typically instituted more quickly than a plan that relies on multiple individual consultations. The clinician and patient benefits of the MDTB include higher quality care in a more coordinated and timely manner. The benefits of MDTB have been recognized by various governing bodies, including the National Institutes of Health, the National Cancer Institute, and the American College of Surgeons (ACS) (4-7). As a testament to the value of MDTB, the ACS created a Commission on Cancer Program accreditation board, requiring that cancer surgery programs have multidisciplinary conferences that prospectively review cases and discuss cancer patients management (8-10).
There is evidence-based support for the clinical value of an MDTB (11-14). Many of the studies that have examined the role of MDTB have originated at large academic institutions, and the outcomes assessed have typically focused on two metrics. The first metric has been adherence to National Comprehensive Cancer Network (NCCN) guidelines before versus after incorporation of an MDTB. Abraham et al. examined the adherence to these guidelines for colorectal cancer (11). The authors reported that patient presentation at a dedicated colorectal MDTB was one of the most important factors directly correlating with delivery of therapy that adhered to NCCN guidelines (OR 3.6, P<0.001). Similarly, Freeman et al. noted improved management with a tumor board (13). In this study that examined patients with esophageal cancer, the authors demonstrated that presentation at a tumor board not only improved adherence to NCCN guidelines, (83% vs. 98%, P<0.001), but also enhanced other surrogate markers of appropriate oncology care including complete staging workup (67% vs. 97%, P<0.001) and mean days from diagnosis to treatment (27 vs. 16, P<0.001). Other studies have reported similar findings, giving credence to the ACS recommendation for the role of the MDTB (15-17).
Additionally, the MDTB has been assessed as a second opinion “service” in which the MDTB recommendations have been compared with outside providers’ (OSPs) treatment recommendations, using the frequency of changed management plans as the measured outcome. One of the first groups to examine this metric was Newman et al. (12). Using a breast MDTB, the authors specifically measured the change in surgical planning before and after consultation. The recommendations of the MDTB were standardized and based on the most recent NCCN guidelines for breast cancer. The authors sub-divided the results according to changes in radiological and pathological interpretation. When looking at differences in interpretation of radiological imaging, 67 (45%) patients had an alteration in interpretation, notably with 43 (29%) patients recommended for additional biopsy, and 6 (4%) patients with suggested residual disease at the site of previous excision. 10 (7%) patients had changes in follow-up recommendations. These changes subsequently led to 16 patients (11%) with a change in surgical planning. In terms of histology, MDTB review with a dedicated breast pathologist resulted in change in diagnosis for 43 of 149 (29%) of patients. Specifically, six patients had their diagnosis changed from ductal carcinoma in situ (DCIS) to lobular carcinoma in situ (LCIS). One patient was upgraded to cancer from benign disease, while two patients had their diagnosis changed from DCIS to invasive/infiltrating cancer. Eight patients (5%) had a change in margin status. In total, this led to a change in surgical management for 13 patients (9%). The authors attributed these differences in management to a collaborative approach based on expert insight (Table 1).
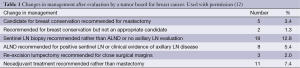
Full table
Some studies have suggested, however, no significant differences in the management of patients when an MDTB is utilized. For example, Riedel et al. investigated a lung cancer MDTB using both time to diagnosis and time to definitive therapy as a marker of efficacy. The authors reported no difference among patients who were and were not evaluated by an MDTB (48 vs. 47 days, P=0.09, and 22 vs. 23 days, P=0.71, respectively). In a separate study, Keating et al. examined the overall impact of MTDB on care delivery (10). These authors surveyed 138 Veterans Affairs (VA) hospitals in the United States and screened for the presence of an MDTB at the institution; the authors then assessed the effect of the MDTB on 27 cancer-specific measures. On multivariate analysis, the presence of an MDTB was not associated with improvements in the predetermined outcome variables for 20 of the 27 measures. Overall, the authors concluded that tumor boards did not influence quality of cancer care and may be an unnecessary allocation of resources. Interestingly, even the authors of other studies in which an MDTB was found not to be associated with improved outcomes have noted that the infrastructure and organization of an MDTB itself are critical to its success. Specifically, the operational details, such as patient-directed involvement, physician “buy-in”, and commitment by all stakeholders are of paramount importance and the lack of these things may have contributed to the VA MDTB’s lack of efficacy (10-18). It is also possible that, because MDTBs often have only one physician in attendance who has actually seen and examined the patient, meaningful exchanges regarding difficult management decisions can be challenging and the input of other physicians who have not meet the patient tend to take on less importance. As such, other multidisciplinary modalities focused more directly at the point of care have been sought.
Multidisciplinary clinics (MDC)
In an attempt to address some of the potential limitations of the MDTB, a new modality has gained momentum in oncology care—the multidisciplinary clinic (MDC). MDCs involve all or most activities of a MDTB, but a major difference is that most or all physicians have a chance to evaluate the patient in person, with access to the same laboratory results, images, and other objective data as the other specialists. Other differences include but are not limited to the stage of presentation of cases, the approach to the patient, the participants involved, and the role of the patient (Table 2). These differences and the highlighted benefits of an MDC over an MDTB have been examined from not just a clinical perspective, but also from an organizational, operations management, and health services context (19,20).
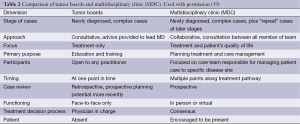
Full table
From an organizational standpoint, the central focus and goals of MDCs have recently been well described. Lemeiux-Charles and McGuire have emphasized the three major goals of the team dynamics of an MDC: (I) there must be full and equal participation of all team members; (II) teams with shared egalitarian values tend to work together effectively; and (III) team coordination improves performance, and the presence of conflict can dilute the coordinated effectiveness of the team (21,22). Without these central goals clearly defined, the authors stress the potential of the MDC will be not reached.
One of the characteristics fundamental to meeting these goals of the MDC and also an integral component of organizational research has been optimizing the team composition of the MDC. In many respects, the members of a MDTB and MDC are similar in terms of the clinical representation—medical oncologists, radiation oncologists, radiologists, pathologists and surgeons, and essential supportive care. But when designing and implementing a successful MDC, the breadth of clinical representatives needs to extend to other support staff, including social workers, mid-level providers, and trainees. Some studies have suggested that the search for appropriate participants should be extended even wider to include primary care physicians, psychologists, and potentially clergy (19,23).
The rationale for including more caregivers in the MDC is based on optimizing the acute medical and psychosocial issues that may arise in the delivery of care, as well as identifying and planning for potential chronic treatment sequelae that may subsequently develop. This is particularly relevant to the delivery of optimal care of patients with cancer as the role of survivorship and surveillance programs are gaining importance (19,24,25). For example, should a patient develop recurrent disease after completion of initial therapy, the primary care physician or oncologist is likely to detect such a recurrence, and if this physician has an already established role in the MDC, then a streamlined process to resume a consensus plan of care is already in place (26-28).
Despite these potential benefits, the inclusion of multiple participants in an MDC does come with challenges. Operationally, too many dissonant voices run the risk of diluting the education and treatment planning aspects of the MDC. Furthermore, with an increased number of clinical participants, there is the potential for inefficient workflows and diminished equanimity. This risk has been described particularly for more complicated tumors that tend to require more participants in MDCs (29). As such, in this scenario there may be a risk between large participant numbers and decreased functioning of complex MDCs, with consequently worse patient outcomes and satisfaction (30). The recommended number of clinicians participating in an MDC has yet to be defined formally, and likely depends on the specific patient population served, and the available infrastructure at a given institution.
The professional expectations of each member involved in the MDC will also vary and must be taken into account when trying to reach the goals of an MDC (31,32). For example, a participating social worker and radiation oncologist have different expectations of professional norms within patients care, as well as team dynamics within their usual occupational environments. If these professional factors are not recognized and accounted for, participants are less likely to adequately contribute their component of multidisciplinary care, impairing the goals of the MDC.
The logistics of the actual location/clinic space where the MDC is to be held also needs to be carefully considered. Options for MDC conference venues include private, clinic-based, or within larger hospital systems. Variation in the quality of delivery of care has been described for all settings, with hospitals typically demonstrating improved MDC functionality (33). Lukas et al. attributed the benefits of the hospital setting to the more neutral environment, in that clinic and private practice settings may predispose to an impression of exclusivity and an atmosphere more conducive to keeping clinicians embedded in their respective “silos” (33). In general, most large academic centers that have MDCs host their MDCs in a centralized hospital setting, thus avoiding this potential for disruption of participant equanimity. Other environmental considerations include rural versus urban location. This has been shown to dramatically influence participants, with the rural setting experiencing more of a primary care influence, compared with the urban setting’s predilection toward specialist and subspecialist practice (19).
The role of improved technology also plays an increased role in the development of MDCs. One such recent advance has been the introduction of the “virtual team” (34-36). These teams integrate telemedicine as an important component of the MDC, increasing the number of participants and perspectives in the MDC. This method of incorporating technology to enhance MDC participation across location boundaries will continue to evolve and will likely serve to improve the collaborative dynamic and limit the influence of location on the MDC. Technological advances have also provided opportunities to improve exposure to potential treatment plans and improve patients’ overall experience. For example, our group and others have recently begun emailing patients prior to their visit with online references to view videos about treatments and expectations for the MDC, which can improve workflow and optimize efficiency.
Outcomes of multidisciplinary clinics
While any malignancy can be considered for evaluation in an MDC, most institutions have found that the role of an MDC is particularly useful for more complex malignancies that require a broad team approach, e.g., pancreas, breast, lung, and liver cancer. These tumors, as well as others, are ideal for MDC use because of the multifaceted input needed from a medical, surgical, radiological, pathological, and social context (37-39).
As with other institutions, given the number of patients referred and the number of clinicians involved, we have found that weekly MDC are most appropriate. The leadership of the MDC is typically shared between the nurse coordinator and clinical director. In turn, the governance model of the MDC should be well delineated and outline the expectations of each participant, the relative interaction with other clinicians and support staff on the MDC, and the roles to be fulfilled at each clinic. The MDC leaders, in conjunction with all participants, need to develop a strategic mission for the MDC, incorporating clinical care, research and organizational goals, as well as objective methods to assess each of these domains.
Since 2005, our group at Johns Hopkins Hospital has had a MDC for pancreas cancer (40). Under the direction of the nurse coordinator, all patients arrive at the MDC by 7:00 am, and they are given a schedule of their encounters for the day. Most patients have completed their evaluation and are with a consensus treatment plan by 4:00 pm (Table 3).

Full table
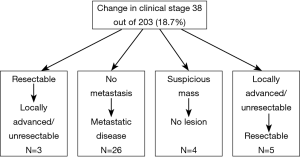
The pancreas MDC has provided several objective benefits in the management of patients with pancreatic cancer. In fact, we reported that almost 1 in 4 patients seen in the pancreas MDC had a change in their recommended management based on the multidisciplinary input of MDC members. For example, one of the greatest influences on alteration in treatment plans involved standardization of imaging performance and interpretation (40). After review at the MDC, many patients were deemed to have inadequate imaging to provide accurate and optimal data to based management plans. Pancreas cancer has historically been difficult to evaluate from a radiographic standpoint, particularly in terms of the determination of metastatic liver disease, as sub-centimeter metastases are poorly visualized on axial imaging (41,42). The determination of accurate local and distant staging is critical to the appropriate management of patients with pancreas cancer because only those patients without metastatic disease are likely to benefit from surgical resection. Remarkably, in our series of 203 patients, 26 of 38 (68.4%) patients were found to have previously undetected metastases, thereby upstaging them to stage IV disease. We attribute this finding to better cross-sectional imaging at our institution, as well as improved interpretation of the images by a dedicated abdominal pancreas radiologist. In addition to specialized radiologists and better resolution cross-sectional imaging, differences in radiologic interpretation may be due to the use of 3D reconstruction to better assess local resectability (43). With better cross-sectional imaging, the relationship of the pancreas to the surrounding vascular structures can be more accurately evaluated. In turn, the surgical consensus regarding whether the pancreas cancer is operable versus inoperable may change. In fact, we have noted that 10-15% of patients seen at our pancreatic MDC were downstaged from originally being considered locally advanced/unresectable to resectable tumors that were subsequently taken to the operating room for resection. It should be noted that this change in management is probably not solely attributable to improved imaging, but rather a result of review and interpretation of imaging between the radiologists and surgeons, highlighting the importance of inter-disciplinary discourse in treatment planning.
Expert pathologist input in the MDC setting can similarly lead to important changes to a patient’s care. In our pancreas MDC, we noted alterations in management based on changes in histological diagnosis. Specifically, 3% of patients had an alteration in diagnosis, including diagnoses that were changed from pancreas adenocarcinoma to metastatic breast (n=1), gastrointestinal stromal tumors (n=1), gallbladder cancer (n=1), serous cystadenoma (n=1), benign inflammatory process (n=1), and well-differentiated endocrine neoplasms (n=2) (Figure 1). These data serve to emphasize the importance of expert pathological input in the MDC setting—as a small subset of patients will have a change in diagnosis based on expert pathological review of biopsy results.
Another benefit of the MDC can be increased enrollment and participation in applicable clinical trials. At our institution, we noted that in the year prior to initiation of the MDC, only 49.2% of the patients seen at individual clinics at our institution with a diagnosis of pancreatic cancer enrolled in the National Familial Pancreas Tumor Registry (NFPTR). In contrast, after initiation of the MDC, enrollment in the NFPTR increased to 77.8%. This led to 51 patients being offered participation in clinical trials, and 10 patients being actively enrolled in trials.
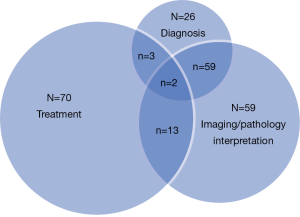
At Johns Hopkins Hospital, we have also initiated an MDC for liver malignancies (39), Similar to our findings with pancreas cancer, we found notable differences in the treatment plans and recommendations generated by OSPs versus recommendations derived by the MDC. Among the 343 patients seen in the liver MDC between 2009 and 2012, we have found that about 8-10% of patients had a change in diagnosis based on imaging. In addition, there was also a change in diagnosis for 10% of patients based on alterations in the pathological review of the specimen by an expert hepato-pathologist. Together, in total, about 40% of patients who were seen in the liver MDC had a change in the diagnosis, imaging interpretation, or therapeutic plan (Figure 2). Yopp et al. has also reported on the results of a liver MDC that had components including triage assessment, multidisciplinary clinical input, and a consensus conference to determine the treatment plan (44). In the study by Yopp et al., the authors compared outcomes of patients before (n=250) versus after (n=105) implementation of their MDC. In comparing these two cohorts, while there was no difference in degree of chronic liver dysfunction, patients in the MDC cohort had earlier-stage tumors, fewer symptoms, and decreased evidence of metastases. The median time to treatment after diagnosis in the MDC period was significantly shorter than in the earlier pre-MDC time period (2.3 vs. 5.3 months, P=0.002). On multivariate analysis, being seen in the MDC remained independently associated with better overall survival (hazard ratio 2.5, 95% confidence interval 2-3), after adjusting for stage and recipient of curative treatment. Patients diagnosed after MDC initiation had a median survival of 13.2 months compared to the 4.8 months observed in patients diagnosed before MDC formation (P=0.005). While baseline differences in the pre- and post-MDC cohorts make definitive conclusions about survival difficult, the authors suggested that the implementation of a MDC for the evaluation and treatment of patients with liver cancer was associated with improved overall survival.
Conclusions
While the concept of multidisciplinary care has gained acceptance at many medical institutions, the optimal mechanism for delivering this multidisciplinary care continues to evolve. Certainly, MDTB play a key role in expanding physician education and thus improving cancer care. However, these tumor boards have limitations, and we believe that the formal MDC represents the best means of multidisciplinary care delivery. Recent data would suggest that this MDC approach not only facilitates convenient, coordinated and patient-centered care, but that the MDC may also improve delivery of care by enhancing diagnostic accuracy in complex malignancies and by developing up to date, evidence-based, personalized care plans for cancer patients. The MDC framework is likely to assist with efficient resource utilization as the health care system moves more towards bundled care. More research is needed in this area, as there is still a lack of consensus as to the appropriate algorithm and format for delivering optimal multidisciplinary care within specific disease sites. However, clinicians and patients alike recognize that the framework of multidisciplinary care has been established in cancer treatment, and this delivery model will continue to evolve with a focus on maximizing safety, improving efficiency, and optimizing patients care.
Acknowledgements
Disclosure: The authors declare no conflicts of interest.
References
- Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: a randomized controlled trial. J Clin Oncol 2006;24:635-42. [PubMed]
- Fleissig A, Jenkins V, Catt S, et al. Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncol 2006;7:935-43. [PubMed]
- Henson DE, Frelick RW, Ford LG, et al. Results of a national survey of characteristics of hospital tumor conferences. Surg Gynecol Obstet 1990;170:1-6. [PubMed]
- Malin JL, Schneider EC, Epstein AM, et al. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol 2006;24:626-34. [PubMed]
- Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: the Quality Oncology Practice Initiative. J Clin Oncol 2005;23:6233-9. [PubMed]
- Petty JK, Vetto JT. Beyond doughnuts: tumor board recommendations influence patient care. J Cancer Educ 2002;17:97-100. [PubMed]
- Warnecke R, Fennell M, Havlicek P. Network demonstration projects and cancer control: the head and neck demonstration networks. Prog Clin Biol Res 1983;120:247-65. [PubMed]
- Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg 2002;87:13-5. [PubMed]
- Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol 2008;26:1893-8. [PubMed]
- Keating NL, Landrum MB, Lamont EB, et al. Tumor boards and the quality of cancer care. J Natl Cancer Inst 2013;105:113-21. [PubMed]
- Abraham NS, Gossey JT, Davila JA, et al. Receipt of recommended therapy by patients with advanced colorectal cancer. Am J Gastroenterol 2006;101:1320-8. [PubMed]
- Newman EA, Guest AB, Helvie MA, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer 2006;107:2346-51. [PubMed]
- Freeman RK, Van Woerkom JM, Vyverberg A, et al. The effect of a multidisciplinary thoracic malignancy conference on the treatment of patients with esophageal cancer. Ann Thorac Surg 2011;92:1239-42; discussion 1243. [PubMed]
- Freeman RK, Van Woerkom JM, Vyverberg A, et al. The effect of a multidisciplinary thoracic malignancy conference on the treatment of patients with lung cancer. Eur J Cardiothorac Surg 2010;38:1-5. [PubMed]
- Croke JM, El-Sayed S. Multidisciplinary management of cancer patients: chasing a shadow or real value? An overview of the literature. Curr Oncol 2012;19:e232-8. [PubMed]
- Lamb BW, Sevdalis N, Mostafid H, et al. Quality improvement in multidisciplinary cancer teams: an investigation of teamwork and clinical decision-making and cross-validation of assessments. Ann Surg Oncol 2011;18:3535-43. [PubMed]
- Haddad P, Mir MR, Jamali M, et al. Gastrointestinal tumor board: an evolving experience in Tehran Cancer Institute. Acta Med Iran 2013;51:270-3. [PubMed]
- Patkar V, Acosta D, Davidson T, et al. Cancer multidisciplinary team meetings: evidence, challenges, and the role of clinical decision support technology. Int J Breast Cancer 2011;2011:831605.
- Fennell ML, Das IP, Clauser S, et al. The organization of multidisciplinary care teams: modeling internal and external influences on cancer care quality. J Natl Cancer Inst Monogr 2010;2010:72-80.
- Zapka JG, Taplin SH, Solberg LI, et al. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev 2003;12:4-13. [PubMed]
- Lemieux-Charles L, McGuire WL. What do we know about health care team effectiveness? A review of the literature. Med Care Res Rev 2006;63:263-300. [PubMed]
- Alexander JA, Lichtenstein R, Jinnett K, et al. Cross-functional team processes and patient functional improvement. Health Serv Res 2005;40:1335-55. [PubMed]
- van Wersch A, Bonnema J, Prinsen B, et al. Continuity of information for breast cancer patients: the development, use and evaluation of a multidisciplinary care-protocol. Patient Educ Couns 1997;30:175-86. [PubMed]
- Morgan MA. Cancer survivorship: history, quality-of-life issues, and the evolving multidisciplinary approach to implementation of cancer survivorship care plans. Oncol Nurs Forum 2009;36:429-36. [PubMed]
- Alfano CM, Ganz PA, Rowland JH, et al. Cancer survivorship and cancer rehabilitation: revitalizing the link. J Clin Oncol 2012;30:904-6. [PubMed]
- Grunfeld E. Primary care physicians and oncologists are players on the same team. J Clin Oncol 2008;26:2246-7. [PubMed]
- Earle CC, Burstein HJ, Winer EP, et al. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol 2003;21:1447-51. [PubMed]
- Mayer EL, Gropper AB, Neville BA, et al. Breast cancer survivors’ perceptions of survivorship care options. J Clin Oncol 2012;30:158-63. [PubMed]
- King M, Jones L, McCarthy O, et al. Development and pilot evaluation of a complex intervention to improve experienced continuity of care in patients with cancer. Br J Cancer 2009;100:274-80. [PubMed]
- Shortell SM, Marsteller JA, Lin M, et al. The role of perceived team effectiveness in improving chronic illness care. Med Care 2004;42:1040-8. [PubMed]
- Leicht KT, Fennell ML. eds. Professional work: A sociological approach. Wiley-Blackwell Publishing, 2001.
- Mick SS, Wyttenbach ME. Advances in health care organization theory. Jossey-Bass Incorporated Pub, 2003.
- Lukas CV, Mohr DC, Meterko M. Team effectiveness and organizational context in the implementation of a clinical innovation. Qual Manag Health Care 2009;18:25-39. [PubMed]
- Axford AT, Askill C, Jones AJ. Virtual multidisciplinary teams for cancer care. J Telemed Telecare 2002;8 Suppl 2:3-4. [PubMed]
- Kunkler IH, Fielding RG, Brebner J, et al. A comprehensive approach for evaluating telemedicine-delivered multidisciplinary breast cancer meetings in southern Scotland. J Telemed Telecare 2005;11 Suppl 1:71-73. [PubMed]
- Kunkler IH, Prescott RJ, Lee RJ, et al. TELEMAM: a cluster randomised trial to assess the use of telemedicine in multi-disciplinary breast cancer decision making. Eur J Cancer 2007;43:2506-14. [PubMed]
- Pawlik TM, Vauthey JN, Abdalla EK, et al. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg 2006;141:537-43; discussion 543-4. [PubMed]
- Evans DB, Crane CH, Charnsangavej C, et al. The added value of multidisciplinary care for patients with pancreatic cancer. Ann Surg Oncol 2008;15:2078-80. [PubMed]
- Zhang J, Mavros MN, Cosgrove D, et al. Impact of a single-day multidisciplinary clinic on the management of patients with liver tumours. Curr Oncol 2013;20:e123-31. [PubMed]
- Pawlik TM, Laheru D, Hruban RH, et al. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol 2008;15:2081-8. [PubMed]
- DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med 2004;141:753-63. [PubMed]
- House MG, Yeo CJ, Cameron JL, et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg 2004;8:280-8. [PubMed]
- Qiu H, Wild AT, Wang H, et al. Comparison of conventional and 3-dimensional computed tomography against histopathologic examination in determining pancreatic adenocarcinoma tumor size: implications for radiation therapy planning. Radiother Oncol 2012;104:167-72. [PubMed]
- Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol 2014;21:1287-95. [PubMed]


