Algorithm for the management of metastatic cutaneous melanoma
Introduction
Over the last several years we have witnessed great progress in the treatment of patients with metastatic melanoma (1). As shown in Figure 1, most of the Food and Drug Administration (FDA) drug approvals took place over the last 4 years. Although metastatic melanoma remains a frequently lethal disease, a large proportion of patients can now be anticipated to respond to therapy and a substantial minority, particularly those receiving immunotherapy, can even be cured.
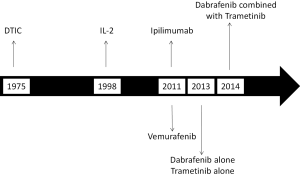
The treatment modalities which are either approved by the FDA or commonly used for the treatment of metastatic melanoma include:
- Chemotherapy-single agents such as dacarbazine (DTIC) and temozolomide, fotemustine (not approved in the US); combination chemotherapy with various regimens such as carboplatin/paclitaxel and the CVD (cisplatin, vinblastine and DTIC) regimen;
- High-dose interleukin-2 (HD IL-2);
- Biochemotherapy (CVD in combination with IL-2 and interferon-alfa);
- Ipilimumab;
- Tumor targeted therapy [BRAF inhibitors (BRAFi) or the combination of BRAF and MEK inhibitors]
The optimal manner of integrating these treatment options and the ideal treatment sequence is not clearly established and remains a matter of great debate. In this review we will briefly summarize the efficacy results of the various treatment options and discuss the algorithms that we favor for the most common clinical scenarios of metastatic disease.
Efficacy of the currently available treatment options
Chemotherapy
DTIC was approved in 1975 based only on overall response rates, which were historically reported in the range of 10% to 20% (2). However, in more recent phase III trials which used DTIC as comparator, the objective response rate by RECIST criteria was only 5% to 10% (2-4). Temozolomide was shown in a phase III trial to have similar efficacy to DTIC (5). Other cytotoxic agents with activity in patients with metastatic melanoma include fotemustine (not approved in the US), cisplatin and carboplatin, the vinca alkaloids, the taxanes and nitrosoureas (2). All these agents result in objective response rates ranging from 5% to 20%. Combination chemotherapy typically results in an increased objective response rate (20% to 30%) compared to single agents, but no improvement in overall survival (2).
The activity of systemic chemotherapy in patients with brain metastases is very limited. A phase II study which evaluated the efficacy of temozolomide in treatment naïve patients with brain metastases showed a response rate of only 7% (6). The response rate of fotemustine was reported to be in the range of 10% to 20% in this patient population; however, responses to either agent were generally short-lived (7).
High-dose IL-2 (HD IL-2)
HD IL-2 was approved by the FDA in 1998, based on the observation that it could produce durable complete remissions in approximately 5% of the patients (8,9). Although the overall response rate is of only 15%, the National Cancer Institute experience suggests that patients with disease primarily restricted to skin and/or lymph nodes have a much higher response rate, approaching 50% (10). HD IL-2 has no effect on brain metastases and, like other forms of immunotherapy, is contra-indicated in patients taking corticosteroids or other immunosuppressive drugs. HD IL-2 has a high acute toxicity and can be administered only in centers with considerable expertise with this treatment modality.
Biochemotherapy
The combination of cisplatin-based chemotherapy, in general the CVD regimen, with IL-2 and interferon-alfa, was coined in the early 1990s as “biochemotherapy”. In phase II studies, it showed overall response rates in the range of 40% to 50%, and resulted in long-term survival in approximately 5% to 10% of the patients (2). The only positive randomized trial, which compared biochemotherapy with chemotherapy, was conducted at the MD Anderson Cancer Center, an institution with a large experience with the use of IL-2-based regimens (11). In this study, a total of 190 patients were randomized to sequential biochemotherapy or chemotherapy with the CVD regimen. The overall response rate was significantly higher with biochemotherapy (48% versus 25%) and there were six complete responders in the biochemotherapy arm versus 2 for the CVD arm. The median time to progression was 4.9 months for the biochemotherapy arm compared with 2.4 months for the CVD arm (P=0.008) (11). Although approximately 50% of the patients on the chemotherapy arm crossed over to subsequently receive IL-2-based regimens, the median overall survival was 11.9 months for the biochemotherapy arm versus 9.2 months for the chemotherapy arm (P=0.03 by two-sided Wilcoxon test and 0.06 by two-sided log-rank test). A total of 14.3% of the patients on the biochemotherapy arm were alive compared with 6.5% for the chemotherapy arm, with a median follow-up greater than 52 months (11).
In contrast, the study conducted by the US intergroup (E3695), which compared concurrent biochemotherapy with the CVD regimen, showed only a numerical increase in response rate (19.5% vs. 13.8%; P=0.14) and a statistically significant increase in progression free survival (4.8 vs. 2.9 months; P=0.015), but no improvement in overall survival (12). It is important to emphasize that in the intergroup study, the protocol called for frequent dose reductions, which were not employed in the MD Anderson protocol, and that the study was conducted by medical oncologists with on average, less experience with IL-2-based therapy. The impact of this inexperience was reflected by the fact that 20% of the patients on the biochemotherapy arm were unevaluable due to protocol violations (12). In our opinion, despite the high toxicity and inability to confirm a survival benefit in the cooperative group setting, concurrent biochemotherapy remains a treatment option in centers which have a large experience with this form of therapy.
Ipilimumab
Ipilimumab is a human monoclonal antibody that blocks the activity of CTLA-4, a down-regulator of T cell function; thus, restoring T cell function for prolonged periods of time. Ipilimumab was approved by the FDA in 2011 for use in patients with advanced melanoma based on two randomized, phase III, studies. The first trial included a total of 676 patients with previously treated, unresectable stage III or stage IV melanoma, who were HLA-A*0201-positive and were randomized to ipilimumab with or without glycoprotein 100 (gp100) peptide vaccine and to the gp100 vaccine plus placebo (13). The median overall survival was 10 and 10.1 months for patients receiving ipilimumab alone or with the gp100 vaccine, respectively, versus 6.4 months for patients receiving the vaccine alone [hazard ratio (HR), 0.68; P<0.001; HR, 0.66; P<0.003]. In a subgroup analysis, there was a 53% reduction in the risk of death for patients with M0, M1a or M1b disease compared with 28% reduction for patients with M1c disease. This study showed for the first time that an immunotherapy could improve overall survival in patients with metastatic melanoma and also suggested that patients with normal LDH derived greater benefit.
The second trial included 502 patients with no prior systemic therapy for metastatic disease and compared in a 1:1 ratio ipilimumab (10 mg/kg) plus dacarbazine (850 mg/m2) or placebo plus dacarbazine (850 mg/m2) (14). The median OS was 11.2 months for the combination [95% confidence interval (CI), 9.4-13.6] versus 9.1 months for dacarbazine alone (95% CI, 7.8-10.5). Estimated survival rates in the two groups respectively were 47.3% and 36.3% at 1 year; 28.5% and 17.9% at 2 years; and 20.9% and 12.2% at 3 years (HR for death with ipilimumab-dacarbazine, 0.72; P<0.001) (14). This trial gave support to the first trial and led to the approval of ipilimumab as a single agent both in first and subsequent lines of therapy for patients with metastatic melanoma in the US and most of Europe. In Brazil and several other countries, ipilimumab is approved only as second line therapy.
A composite analysis of 12 clinical studies confirmed the potential long term survival impact of ipilimumab. In this series, 1,257 patients were pretreated and 604 were previously untreated for metastatic disease (15). The dose of ipilimumab was 3 mg/kg for 965 patients and 10 mg/kg for 706 patients. The median overall survival for the whole patient population was 11.4 months. Most importantly, the survival curve reached a plateau of 22% at 3 years, which extended to 10 years, and it was independent of the dose (15).
A phase II trial specifically evaluated the activity of ipilimumab in patients with brain metastases and showed an overall response rate of 15.7% (n=51) in patients not on corticosteroids (16). Responses were rare in a cohort of patients who were on corticosteroids at time of treatment initiation. In a retrospective analysis of the Italian Expanded Access Program, an overall response rate of 11% was reported in a total a 146 patients with brain metastases (17). In a retrospective analysis of the French Expanded Access Program, 3 out of 38 patients responded (18). Patients on corticosteroids (exceeding prednisone 10 mg/d or equivalent) are generally felt to be unlikely to benefit from ipilimumab therapy, although occasional responses have been noted.
Tumor targeted therapy
BRAF inhibitor
There are currently two BRAF inhibitors available on the market: vemurafenib and dabrafenib. The approval of vemurafenib occurred in 2011, the same year that ipilimumab was approved. Its approval was based on an international, multicenter trial called BRIM-3 that screened 2,107 patients with previously untreated, stage IIIC or IV melanoma for the BRAF V600 mutation and identified 675 patients by the Cobas® 4800 BRAF V600 Mutation Test (3). Patients were randomly assigned to receive either vemurafenib (960 mg orally twice daily) or dacarbazine (1,000 mg/m2 intravenously every 3 weeks). At the initial planned interim analysis, the Data and Safety Monitoring Board determined that both the overall survival and progression free survival endpoints had met the prespecified criteria for statistical significance in favor of vemurafenib and recommended that patients in the dacarbazine group be allowed to crossover to receive vemurafenib. The overall survival in the vemurafenib arm was clearly superior to that in the dacarbazine arm. In this initial analysis, the HR for death in the vemurafenib group was 0.37 (95% CI, 0.26-0.55; P<0.001). The HR for tumor progression in the vemurafenib arm was 0.26 (95% CI, 0.20-0.33; P<0.001). The estimated median PFS was 5.3 versus 1.6 months in the vemurafenib and dacarbazine arms, respectively. In a post-hoc analysis, the benefit of vemurafenib relative do dacarbazine was greater in patients with stage M1c disease, especially those with an increase in serum lactate dehydrogenase levels (3).
In the most recent update of this trial, median overall survival continued to be significantly longer in the vemurafenib group than in the dacarbazine group [13.6 months (95% CI, 12.0-15.2) vs. 9.7 months (7.9-12.8); HR 0.70 (95% CI, 0.57-0.87); P=0.0008], as was median progression-free survival [6.9 months (95% CI, 6.1-7.0) vs. 1.6 months (1.6-2.1); HR 0.38 (95% CI, 0.32-0.46); P<0.0001] (19). For the 598 (91%) patients with BRAF (V600E) disease, median overall survival in the vemurafenib group was 13.3 months (95% CI, 11.9-14.9) compared with 10.0 months (8.0-14.0) in the dacarbazine group [HR 0.75 (95% CI, 0.60-0.93); P=0.0085]; median progression-free survival was 6.9 months (95% CI, 6.2-7.0) and 1.6 months (1.6-2.1), respectively [HR 0.39 (95% CI, 0.33-0.47); P<0.0001]. For the 57 (9%) patients with BRAF (V600K) disease, median overall survival in the vemurafenib group was 14.5 months (95% CI, 11.2-not estimable) compared with 7.6 months (6.1-16.6) in the dacarbazine group [HR 0.43 (95% CI, 0.21-0.90); P=0.024]; median progression-free survival was 5.9 months (95% CI, 4.4-9.0) and 1.7 months (1.4-2.9), respectively [HR 0.30 (95% CI, 0.16-0.56); P<0.0001]. A phase II trial of vemurafenib in previously treated patients yielded similar efficacy results suggesting that exposure to prior therapy did not affect vemurafenib’s antitumor activity (20).
Dabrafenib was approved in 2013 based on a randomized trial that compared dabrafenib with dacarbazine, called BREAK-3. In this study, a total of 250 patients with unresectable stage III or IV melanoma and BRAF V600E mutations were randomly assigned in a 3:1 ratio to dabrafenib 150 mg orally twice a day or dacarbazine 1,000 mg/m2 IV every 3 weeks (4). IL-2 was allowed as prior treatment for advanced disease. The primary endpoint was progression-free survival and patients could cross over at the time of progressive disease after confirmation by a blinded Independent Review Committee. The HR for progression-free-survival was 0.30 (95% CI, 0.18-0.51; P<0.0001) favoring dabrafenib over dacarbazine. The estimated median progression-free-survival was 5.1 versus 2.7 months for dabrafenib and dacarbazine, respectively. The overall survival data are limited by the median duration of follow up and crossover. Partial response rates were 47% and 5%, and CR rates were 3% and 2% in patients receiving dabrafenib versus dacarbazine, respectively (4). The results of the BREAK-3 trial are very similar to those of the BRIM-3 trial.
BRAF inhibitors have also shown activity in patients with CNS metastases. In a multicenter phase II study, 172 patients with asymptomatic brain metastases containing either the V600E or V600K mutation were treated with dabrafenib (21). In the 139 patients whose tumor contained a V600E mutation and whose brain metastases were treatment naïve, objective responses were observed in 29 of 74 patients (39%). The response rate in those who had received prior local treatment was 31% (20 of 65). Objective responses in the CNS were observed in 5 of 33 patients (15%) with tumors containing a V600K mutation. The activity of vemurafenib in the CNS was evaluated in a smaller phase II study (22). A total of 18 patients were treated: 9 with no prior therapy to the brain (group A) and 6 with previous surgery and/or radiotherapy with residual disease (group B); 3 patients had prior “brain therapy” but with evidence of progression in CNS before the start of vemurafenib and were included in group A (22). The overall response rate was 50% for both groups, an activity similar to dabrafenib.
MEK inhibitor
Trametinib is the only MEK inhibitor available on the market and was approved in 2013, contemporaneous with dabrafenib’s approval. It selectively inhibits MEK1 and MEK2. As MEK is a downstream event from BRAF, dabrafenib is active in tumors that contain a BRAF mutation. The approval of trametinib was based on a randomized phase III study called METRIC (23). In this study, a total of 1,022 patients were screened for BRAF mutations, resulting in 322 eligible patients (281 with V600E, 40 with V600K and 1 with both mutations). Patients were randomly assigned in a 2:1 ratio to receive trametinib (2 mg once daily) or IV chemotherapy (either dacarbazine 1,000 mg/m2 every 3 weeks or paclitaxel 175 mg/m2 every 3 weeks). Crossover was allowed, and the primary endpoint was progression-free-survival. The investigator-assessed progression-free-survival was 4.8 months in patients receiving trametinib versus 1.5 months in the chemotherapy group (HR for PFS or death, 0.45; 95% CI, 0.33-0.63; P<0.001). Median OS had not yet been reached at the time of last analysis (23).
Combination of BRAF and MEK inhibitor
Resistance to BRAF inhibitors, in patients with BRAF V600 mutations, may be associated with reactivation of the MAP kinase pathway (24). Early phase I/II data with combinations of BRAF and MEK inhibitors showed promising results. More recently, a randomized phase II study that evaluated trametinib in combination with dabrafenib or dabrafenib plus placebo until disease progression was reported (25). Results showed that 76% of participants treated with trametinib in combination with dabrafenib had objective responses that lasted a median of 10.5 months. In contrast, 54% of participants treated with dabrafenib plus placebo experienced objective responses that lasted an average of 5.6 months (25). At the ASCO 2014, the results of the phase III trial, called COMBI-d, which compared the combination of dabrafenib plus trametinib with dabrafenib plus placebo was presented (26). A total of 423 patients were randomized. With a median follow-up of 9 months, there was an increase in the median progression free survival from 8.8 to 9.3 months (HR 0.75; 95% CI, 0.57-0.99; P=0.035) in favor of the combination. There was also an improvement in response rate (67% versus 51%). It is too early for a final analysis of the overall survival but the interim analysis favors the combination. In terms of toxicity, there was more pyrexia but significantly less cutaneous toxicity with the combination. These data lead to the approval of the combination dabrafenib and trametinib by the FDA. Considering the toxicity profile and efficacy, the combination is now the preferred strategy in patients with BRAF mutant melanoma in the US.
Definition of tumor burden
Tumor burden refers to the total amount of cancer tissue in the body. The survival of a patient relates to the tumor burden, disease location and, most importantly, the pace of the disease. In general, patients with high tumor have a high pace of disease and, therefore, a very short survival without therapy. On the other hand, patients with low tumor burden usually have low pace disease and long survival even without therapy. However, these are only the extremes of the disease spectrum, which encompasses a range of clinical scenarios.
High tumor burden will be defined in this paper as large volume of total disease identified by imaging studies in patients who have disease related symptoms and/or elevated serum LDH. Low tumor burden will be defined as low volume disease by imaging studies, with minimal or no symptoms and normal serum LDH.
Treatment algorithms
In medicine, it is not possible to design precise algorithms. In this review, our goal is to focus on the most common clinical settings, while acknowledging that many gray areas will not be covered in our discussion. We envision five distinct scenarios:
- Patients with low systemic tumor burden and no evidence of central nervous system (CNS) involvement;
- Patients with low systemic tumor burden and minor involvement of the CNS;
- Patients with high systemic tumor burden and no involvement of the CNS;
- Patients with high systemic tumor burden and extensive involvement of the CNS;
- Patients with extensive involvement of the CNS and with low or no systemic disease.
There are to date very limited data or recommendations on sequencing of therapies in patients with metastatic melanoma, particularly in patients with BRAF mutated melanomas (27). One retrospective experience addressing this point was recently reported by Ascierto et al. (28). Of 93 patients with BRAF (V600) mutation-positive advanced melanoma who received vemurafenib or dabrafenib before (n=45) or after (n=48) treatment with ipilimumab 3 mg/kg, the median overall survival from first treatment was 9.9 and 14.5 months, respectively. Among patients treated with a BRAF inhibitor first, median survival from the end of BRAF inhibitor therapy was 1.2 months for those who did not complete ipilimumab treatment as per protocol, compared with 12.7 months for those who did (P<0.001). Similarly Ackerman et al. (29) reported in a retrospective analysis of 274 patients that BRAF inhibitor (BRAFi) therapy was equally effective in patients with prior immunotherapy as those who were treatment naïve, while ipilimumab was essentially inactive in the 32 patients who had received prior BRAF inhibitor therapy. Median overall survival was 5 months in this group of patients and the only patients still alive at 1 year were those that at stopped BRAF inhibitor therapy due to toxicity and were back on this treatment approach. While these data suggest that the use of ipilimumab before BRAF inhibitors in patients with BRAF mutated melanoma may be the preferred approach, the differences in outcome are likely, at least in part, due to a bias in patient selection. In particular patients with more aggressive disease were more likely to received BRAF inhibitor therapy first rather than ipilimumab. Prospective randomized studies are required to determine the optimal sequencing of ipilimumab and BRAF inhibitors in patients with BRAF-mutated metastatic melanoma. To address this concern the US intergroup has proposed a trial in which patients are randomly assigned to receive either BRAFi/MEKi therapy followed by ipilimumab based immunotherapy at progression or the converse treatment sequence (EA6134).
Patients with low systemic tumor burden and no evidence of CNS involvement
For patients with low systemic tumor burden and no evidence of CNS involvement, we favor ipilimumab as first option even in patients with BRAF mutated melanoma. Figure 2 illustrates this approach. Our choice is justified by the long-term potential impact of ipilimumab of approximately 20%, its favorable toxicity profile and the fact that this subgroup of patients is more likely to benefit from ipilimumab therapy.
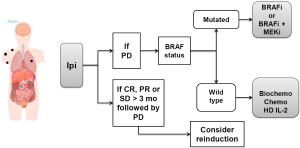
Although the use of BRAFi or BRAFi plus MEKi is also a solid option in first line as it improves overall survival, in case of rapid progression after this tumor targeted therapy, the later use of ipilimumab may not be possible, thus, precluding the patient from receiving a drug that may result in long-term treatment free survival. If the patient achieves an objective response or stable disease longer than 3 months from the last dose of ipilimumab and then exhibits disease progression, reinduction with ipilimumab should be considered in countries in which this approach is approved (e.g., Brazil, USA). In patients who fail to respond to ipilimumab, the subsequent treatment decision should be based on the BRAF status of the tumor. If mutated, the patient should receive BRAFi or BRAFi plus MEKi (combination is favored if approved). If the tumor is not mutated, the patient should be considered for HD IL-2, biochemotherapy or systemic chemotherapy with single agent or combination chemotherapy depending on the patient’s age and comorbidities.
Patients with low systemic tumor burden and minor involvement of the CNS
In this setting, we favor one of two options: treat with stereotactic radiosurgery (SRS) and start ipilimumab or administer ipilimumab and monitor closely the CNS with MRI of the brain in 6 weeks (Figure 3). In case of an increase in the CNS lesions, treat with SRS and continue with ipilimumab. The later approach is warranted as ipilimumab has some activity in brain metastases. However, as the antitumor effect of ipilimumab in the brain is modest (in order of 10% to 15%), close monitoring is mandatory to avoid the patient from requiring corticosteroid therapy. Of note, ipilimumab can cause pseudoprogression of disease including in the CNS and, therefore, it may be permissible to continue to delay CNS radiation in some patients with limited disease progression who remain asymptomatic.

If the patient does not respond to ipilimumab, the subsequent treatment will depend on the BRAF status. If the tumor is BRAF mutated, the patient should receive BRAFi or BRAFi plus MEKi (the combination is favored if approved). If the tumor is BRAF wild type and the CNS is completely controlled, one may consider in young patients with no comorbidities, HD IL-2 or biochemotherapy. Otherwise, systemic chemotherapy with temozolomide, fotemustine, CVD and carboplatin/paclitaxel should be offered.
Patients with high systemic tumor burden and no involvement of the CNS
In patients with high systemic tumor burden and no involvement of the CNS, the BRAF status of the tumor is of critical importance as the highest response rates are observed in patients with BRAFi or BRAF plus MEKi (Figure 4). Furthermore, the responses with tumor-targeted therapy are extremely rapid, resulting in prompt improvement of the patient’s clinical condition.

Ipilimumab, on the other hand, would not be recommended as first line in this setting, as the response rate is low (approximately 10% to 15%) and it occurs more gradually. Patients that respond to BRAFi or the combination of BRAFi plus MEKi should be followed very closely with imaging studies in order to detect very early progression of disease. In this setting of lower tumor burden, they should then be considered for ipilimumab therapy in an attempt to achieve long term survival. In patients with high tumor burden and wild type tumor, one of the best options for rapid cytoreduction is biochemotherapy, which, despite its high toxicity, has an overall response rate of approximately 50%. Other options to consider include systemic chemotherapy. Patients who respond to chemotherapy or biochemotherapy should be considered for ipilimumab therapy at the time of minimal disease progression.
Patients with high systemic tumor burden and extensive involvement of the CNS
In patients with high tumor burden both systemic and in the CNS, the BRAF status of the tumor is also of great importance as the use of BRAFi or the combination of BRAFi and MEKi results in the highest remission rates both for systemic and CNS disease (Figure 5).
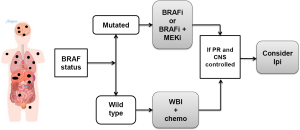
Whole brain irradiation (WBI) has an objective response rate of less than 20%, in more recent studies the response rate is <5%) which is clearly inferior to the response rate associated with BRAFi of approximately 40% to 50% (similar to the systemic response). The safety of the combination of radiation therapy and BRAFi has not been established and some patients have been reported to have significant radiation toxicity when it is administered concomitant with BRAFi therapy. Residual disease may be treated with stereotactic radiation, but typically patients should have an at least 1 week treatment break pre and post WBI. As stated in the previous clinical scenario, responding patients should be followed very closely as they should start ipilimumab at the earliest sign of disease progression, particularly if they are able to get off of immunosuppressive therapy. Patients who have BRAF WT tumors should get SRS to as much tumor as possible followed, if possible, by ipilimumab.
Patients with extensive involvement of the CNS and with low or no systemic disease
In these clinical scenario, the CNS is the most critical site of involvement and most likely responsible for the patient’s imminent death (Figure 6). Thus, the treatment should focus on the CNS. The BRAF status dictates the initial approach as the antitumor activity of the BRAFi (and the combination of BRAFi plus MEKi) is much higher for the CNS than any other treatment modality. Therefore, if the tumor is BRAF mutated, the patient should receive initially a BRAFi or the combination BRAFi plus MEKi. In case of disease progression, the patient should receive focused radiation therapy and continue on BRAF inhibitor therapy.
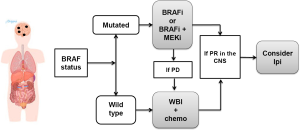
If the patient’s tumor is BRAF wild type, the patient should be treated with SRS and either ipilimumab or chemotherapy as first line. In case of an excellent response in the CNS with either BRAFi or WBI plus chemotherapy, the patient should be followed closely and be considered for ipilimumab at the earliest sign of disease progression, provided that the patient does not require high dose of corticosteroids (exceeding prednisone 10 mg/d or equivalent).
Acknowledgements
Disclosure: Antonio C. Buzaid: Consultancies (GSK, BMS, MSD/Merck, Roche), Honoraria (BMS, Roche); Sanjiv S. Agarwala, Consultancies (GSK, MSD/Merck, Roche), Honoraria (GSK, MSD/Merck, BMS, Roche); Axel Hauschild, Consultancies (GSK, MSD/Merck, Roche), Honoraria (GSK, MSD/Merck, BMS, Roche); Michael B. Atkins, Consultancies (GSK, BMS, MSD/Merck, Roche), Honoraria (GSK, MSD/Merck, BMS, Roche).
References
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet 2014;383:816-27. [PubMed]
- Buzaid AC. Management of metastatic cutaneous melanoma. Oncology 2004;18:1443-50; discussion 1457-9. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [PubMed]
- Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000;18:158-66. [PubMed]
- Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol 2004;22:2101-7. [PubMed]
- Jacquillat C, Khayat D, Banzet P, et al. Chemotherapy by fotemustine in cerebral metastases of disseminated malignant melanoma. Cancer Chemother Pharmacol 1990;25:263-6. [PubMed]
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. [PubMed]
- Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011;17:4550-7. [PubMed]
- Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol 2001;19:3477-82. [PubMed]
- Eton O, Legha SS, Bedikian AY, et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol 2002;20:2045-52. [PubMed]
- Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 2008;26:5748-54. [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New Engl J Med 2011;364:2517-26. [PubMed]
- Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in metastatic or locally advanced, unresectable melanoma. ESMO Annual Meeting 2013:abstr LBA24.
- Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012;13:459-65. [PubMed]
- Queirolo P, Spagnolo F, Ascierto PA, et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol 2014;118:109-16. [PubMed]
- Konstantinou MP, Dutriaux C, Gaudy-Marqueste C, et al. Ipilimumab in melanoma patients with brain metastasis: a retro-spective multicentre evaluation of thirty-eight patients. Acta Derm Venereol 2014;94:45-9. [PubMed]
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323-32. [PubMed]
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707-14. [PubMed]
- Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:1087-95. [PubMed]
- Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer 2014;50:611-21. [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. New Engl J Med 2012;367:107-14. [PubMed]
- Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res 2014;20:1965-77. [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [PubMed]
- Long GV, Stroyakovsky DL, Gogas H, et al. COMBI-d: A randomized, double-blinded, Phase III study comparing the combination of dabrafenib and trametinib to dabrafenib and trametinib placebo as first-line therapy in patients (pts) with unresectable or metastatic BRAF mutation-positive cutane. J Clin Oncol 2014;32:abstr 9011.
- Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol 2013;14:e60-9. [PubMed]
- Ascierto PA, Simeone E, Sileni VC, et al. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: data from the Italian cohort of the ipilimumab expanded access program. Cancer Invest 2014;32:144-9. [PubMed]
- Ackerman A, Klein O, McDermott DF, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer 2014;120:1695-701. [PubMed]


