Mantle cell lymphoma-management in evolution
Mantle cell lymphoma (MCL) is a unique subtype of non-Hodgkin lymphoma (NHL). It accounts for approximately 3-6% of all NHLs, with a median age at diagnosis of 68 years and a significant male predominance (1,2). A majority of patients present with extranodal disease, often involve the gastrointestinal tract, with other clinical features that include splenomegaly, bone marrow involvement and widespread lymphadenopathy (3). The hallmark of MCL is t[11,14](q13;q32). This translocation leads to the overexpression of cyclin D1, leading to dysregulation of the cell cycle (4).
Prognosis can be estimated with clinical factors and with biologic factors. The MCL International Prognostic Index (MIPI) score has been found to be a better predictor of overall survival than the IPI score. Using four independent prognostic factors: lactate dehydrogenase (LDH), white blood cell (WBC) count, age and performance status (PFS), patients are divided into three risk groups. The low risk group encompasses 44% of patients (median OS not reached), with 35% of patients in the intermediate risk group (median OS 51 months) and 21% of patients in the high risk group (median OS 29 months). Additional prognostic factors include the Ki-67 or cell proliferative index (5,6).
There are multiple therapeutic options for patients with newly diagnosed MCL, depending on age and comorbidities. Therapy for patients with relapsed MCL is a rapidly evolving area of study.
Management of newly diagnosed MCL
Most patients with MCL have historically been treated immediately upon diagnosis given the generally unfavorable prognosis. MCL is a clinically heterogeneous disease as evidenced by gene expression profiling showing several unique proliferation signatures correlating to a wide range in survival (7). Investigators at Weill Cornell Medical College in New York evaluated the so-called “watch-and-wait” strategy in 97 patients with newly diagnosed MCL. Based on a time-to-treatment cutoff of >3 months, patients were divided into observation (n=31) and early treatment (n=66) cohorts. Of the 31 patients in the observation cohort, 71% were observed for at least 6 months and 45% were observed for at least 1 year. OS was statistically superior in the observation cohort (likely related to the fact that these patients had better disease characteristics-tended to be younger with lower MIPI scores) (8). This shows that adopting a watch-and-wait strategy can be safely done in a selected group of patients.
Almost all patients will eventually require treatment however. Treatment strategies can be generally divided into intensive and non-intensive therapy for medically fit and medically unfit patients respectively. Most trials of intensive strategies have been limited to patients ≤65 years old without significant co-morbidities (Table 1). Examples of intensive therapies include chemoimmunotherapy induction followed by autologous stem cell transplantation (ASCT) or the R-hyperCVAD (rituximab and hyper-fractionated cyclophosphamide, doxorubicin, vincristine, dexamethasone) with alternating R-high dose cytarabine/methotrexate (R-MA) regimen (Figure 1). Most studies of non-intensive strategies focus on older MCL patients or include patients with significant co-morbidities. Examples of non-intensive combination chemoimmunotherapy regimens include: R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), FCR (fludarabine, cyclophosphamide, rituximab), modified R-hyperCVAD and BR (bendamustine and rituximab) (Table 2).
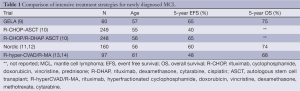
Full table

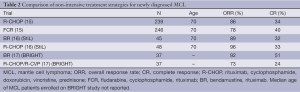
Full table
Therapy for younger, fit patients
Intensive treatments for young, fit patients given without a consolidative ASCT have been studied in MCL. A single institution prospective study from the MD Anderson Cancer Center in Texas evaluated combination therapy with R-hyperCVAD/R-MA. Ninety-seven patients with previously untreated MCL were treated with this regimen. The response rates were high, but the toxicity of this regimen was substantial and the authors did not recommend this as a treatment strategy in patients >65 years old (13). After a median follow-up of 8 years, the median OS had not been reached and the median time to failure (TTF) for the entire study population was 4.6 years with a median TTF of 5.9 years in patients ≤65 years old which was a statistically significant difference (P=0.003) (14). These results were confirmed in a phase II cooperative group study by the Southwest Oncology Group (SWOG 0213) in which 49 patients with advanced, untreated MCL (median age 57 years) were treated with R-hyperCVAD/R-MA. The 3-year PFS and OS were 66% and 81% respectively, with poorer outcomes in those patients >65 years old. Thirty-nine percent of the patients could not complete therapy due to toxicity (18). These studies indicate that the conventional R-hyperCVAD regimen can be prohibitively toxic for most older patients, though can produce durable remissions in younger patients.
Consolidative ASCT can be added to frontline chemoimmunotherapy. This strategy has been evaluated in several European studies. The Nordic Lymphoma Group performed a phase II study of three cycles of dose-intensified CHOP (maxi-CHOP) alternating with three cycles of high-dose cytarabine with rituximab followed by consolidation with an ASCT in 160 patients (145 patients went on to receive ASCT) with MCL. As expected, toxicity was significant, as 17% and 12% of patients were hospitalized for grades 3 and 4 adverse events respectively, mostly neutropenic fever. The 4-year PFS was 73% and the 4-year OS was 81%. After 6 years of follow-up, the median OS was still not reached, while the median event-free survival (EFS) was 7.4 years with a distinct pattern of late relapses noted, especially in those with high risk disease based on MIPI score and Ki-67 index (11,12).
Another European study from GELA evaluated an induction strategy of 3 cycles of CHOP followed by 3 cycles of DHAP (dexamethasone, cytarabine, cisplatin) with rituximab given on cycles 3-6 followed by consolidative ASCT in 60 patients with untreated MCL. The 5-year EFS and OS was 64% and 75% respectively (9). These results were confirmed in a randomized phase III study performed by the European MCL Network in which 497 patients were randomized to induction therapy with R-CHOP ×6 vs. R-CHOP ×3 alternating with R-DHAP ×3 followed by a myeloablative conditioning regimen (included cytarabine for the patients receiving R-DHAP) and ASCT. The CR/CRu rate was significantly higher in the cytarabine containing arm (40% vs. 54%, P=0.0003). OS was also significantly better in the cytarabine arm (NR vs. 82 months, P=0.045) (10). The European studies, when taken together, suggest improved outcomes when high-dose cytarabine is added to the induction regimen.
Therapy for older, less fit patients
There are several non-intensive chemotherapy regimens that can be used for older patients or patients with comorbidities. A study from the European MCL Network was one of the few studies that show the superiority of one treatment strategy over another. In this study, patients who were >60 years old with newly diagnosed MCL were randomized to receive induction therapy with either FCR or R-CHOP. Due to increases in toxicity and a significantly worse OS (4-year OS, 47% vs. 62%, P=0.005), the FCR arm was closed early by the independent data and safety monitoring board, allowing one to conclude that R-CHOP was superior to FCR in MCL patients (15).
Bendamustine is an alkylating chemotherapeutic agent that is active in several subtypes of NHL. It has a more favorable side-effect profile than traditional anthracycline-based chemotherapy regimens, making it ideal for use in the older MCL population. Two randomized studies have compared R-CHOP to BR in patients with previously untreated MCL. The North American BRIGHT study compared BR to both R-CHOP/R-CVP in previously untreated patients with indolent NHL or MCL. Of 447 randomized patients, 74 had MCL. The primary end-point was to demonstrate non-inferiority of the CR rate of BR compared to R-CHOP/R-CVP. In the cohort of MCL patients, the CR rate for BR was statistically superior to R-CHOP/R-CVP (51% vs. 24%, P=0.0180). Time-to-event data is still being collected at this time and has not been reported (17). The Study group indolent Lymphomas (StiL) performed a randomized phase III non-inferiority study of BR compared to R-CHOP in 549 previously untreated patients with advanced indolent lymphoma or MCL. Ninety-four patients had MCL. The primary end-point of PFS was statistically superior in the BR arm for almost all sub-types including MCL (35.4 vs. 22.1 months, P=0.0044). There was also significantly less grade 3-4 leukopenia (P<0.0001), alopecia (P<0.0001) and infectious episodes (P=0.0025) among others in the BR arm (16). Interestingly, there was more GI toxicity seen in the BR arm in the BRIGHT study than seen in the StiL trial. When taken together, these two studies suggest that BR may be superior to R-CHOP in MCL patients.
Bortezomib is a proteasome inhibitor that was initially approved in the R/R setting for MCL. It has made its way to the frontline setting and has been studied in combination with standard chemoimmunotherapy. Bortezomib was combined with the modified R-hyperCVAD regimen (VcR-CVAD). In a phase II study within the Eastern Cooperative Oncology Group (E1405), 75 patients with previously untreated MCL received VcR-CVAD for 6 cycles. The ORR was 95% (95% CI, 87-99%), with a CR rate of 68% (95% CI, 57-79%). The 3-year PFS and OS were 72% and 88% respectively. These response rates compare favorably to many of the intensive strategies used in younger patients. No grade 3/4 peripheral neuropathy was noted in this study, which used lower dosing of bortezomib and vincristine (1.3 mg/m2 and 1 mg respectively) (19). Another phase I/II study combined bortezomib with R-CHOP. Thirty-six patients with previously untreated MCL received R-CHOP plus escalating doses of bortezomib (0.7, 1 or 1.3 mg/m2). The ORR was 81% in the intent-to-treat (ITT) population with 64% CR/CRu. The 2-year PFS was 44% (95% CI, 27-60%) and the 2-year OS was 86% (95% CI, 70-94%). These results suggest an improvement in the CR/CRu rate when bortezomib is combined with R-CHOP (20).
Given that MCL is a disease where relapse is common despite the high initial response rates, maintenance strategies have been studied to see if remission duration can be improved. The E1405 study evaluated 2 years of maintenance rituximab (MR) after VcR-CVAD induction therapy. The decision on whether to pursue MR or ASCT was left to the discretion of the treating physician. With a median follow-up of 4.5 years, the 3-year PFS and OS for the entire study population was 72% (95% CI, 62-84%) and 88% (95% CI, 81-96%) respectively. After adjusting for MIPI risk score and quality of response to induction therapy (PR vs. CR), no statistically significant difference in PFS or survival outcomes was found between the MR and ASCT groups (19). The study by the European MCL Network that compared FCR to R-CHOP as an induction strategy had a second randomization for patients that responded to therapy to either interferon or rituximab. Patients in the interferon arm could receive either standard interferon alpha (3 million units 3 times/week) or pegylated interferon alfa (1 µg/kg/week) and patients in the rituximab arm received rituximab 375 mg/m2 every 2 months until disease progression. When analyzing the cohort of patients that received induction therapy with R-CHOP who went on to receive maintenance treatment, there was a significant difference in the 4-year OS favoring MR (87% vs. 63%, P=0.005) (15). These studies suggest a clear role for MR in older patients responding to induction treatment. Further studies that compare ASCT to MR in a randomized fashion for younger patients should be a consideration in the future.
Management of patients with R/R MCL
There have been several novel agents that have emerged to treat patients with R/R MCL (Table 3). These agents target various steps involved in the B-cell receptor (BCR) signaling pathway (Figure 2).
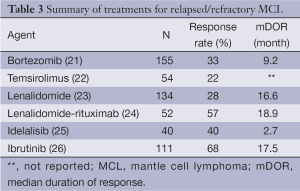
Full table
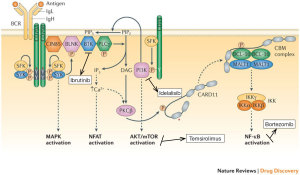
Activation of the anti-apoptotic nuclear factor-kappa B (NF-κB) pathway plays an important role in the pathogenesis of MCL making bortezomib a rational drug for study in this disease. The PINNACLE study was the pivotal trial that led to FDA approval of bortezomib for R/R MCL in 2006. In this phase II study, 155 patients were treated with bortezomib 1.3 mg/m2 IV on days 1, 4, 8 and 11 of a 21-day cycle, with 141 patients assessable for response. The median number of prior therapies was one. The ORR was 33% (8% CR/CRu) and the median duration of response (DOR) was 9.2 months. Grade 3 or higher peripheral neuropathy was seen in 13% of patients (21).
The t[11,14](q13;q32) leads to overexpression of cyclin D1, which is a major pathogenic determinant in MCL. Translation of cyclin D1 is regulated by the mammalian target of rapamycin, or mTOR, kinase, which is itself part of the phosphatidylinositol-3-kinase (PI3K)/AKT intracellular pathway. The mTOR inhibitor temsirolimus was therefore evaluated in R/R MCL. In a phase III study, 162 heavily pretreated patients underwent a 1:1:1 randomization to receive temsirolimus 175 mg weekly for 3 weeks followed by either 75 or 25 mg weekly or a therapy of the investigator’s choice [most common choices were gemcitabine (42%) or fludarabine (23%)]. The primary end-point of PFS was significantly longer in the temsirolimus 175/75 mg group compared to the investigator’s choice group (median PFS, 4.8 vs. 1.9 months, P=0.0009). This study eventually led to the approval of temsirolimus in R/R MCL in Europe (22).
Lenalidomide is an immunomodulatory drug that has multiple effects on the immune system. The EMERGE study evaluated the efficacy of single-agent lenalidomide in 134 heavily pretreated patients with R/R MCL who had failed treatment with bortezomib. Lenalidomide was given orally at 25 mg/day on days 1 through 21 (28 day cycle) and was administered until disease progression or intolerance. The ORR was 28% with 7.5% CR/CRu. The median PFS was just 4 months but the median response duration was 16.6 months, suggesting substantial clinical benefit in the patients that did respond to treatment. The most common grades 3-4 AEs were neutropenia (43%) and thrombocytopenia (27%) with 34% and 31% of patients experiencing fatigue and diarrhea of any grade respectively. It is important to note that treatment was discontinued in 19% of patients due to AEs (23).
Lenalidomide has also been shown to be synergistic with rituximab in several preclinical studies (27,28). A I/II study from the MD Anderson Cancer Center in Texas, studied the combination of lenalidomide plus rituximab (375 mg/m2 weekly ×4 during cycle 1) in 52 patients with R/R MCL (50 patients received lenalidomide at the maximum tolerated dose of 20 mg). The ORR amongst the phase II cohort was 57% (36% CR). The median DOR was 18.9 months with a median PFS of 11.1 months, confirming the possible synergistic effects seen with this combination in vitro. Grades 3-4 neutropenia and thrombocytopenia occurred in 66% and 23% of patients respectively with 52% of patients needing at least one dose reduction or interruption due to AEs (24).
Another important mediator of the BCR pathway is Bruton’s tyrosine kinase (BTK). Ibrutinib is an oral BTK inhibitor designed to block the constitutive activation of the BCR pathway in malignant B-cells. In a recent phase II study, 111 patients with R/R MCL, were given ibrutinib at a dose of 560 mg daily until disease progression or unacceptable toxicities. The median number of cycles received was 9 (range, 1 to 24). The ORR was 68% (21% CR) with a median DOR of 17.5 months and median PFS of 13.9 months. The median PFS for those that achieved a PR or CR was 17.5 months and not reached respectively. Treatment was well-tolerated as only 16% and 11% experienced grades 3-4 neutropenia and thrombocytopenia respectively. Importantly, treatment was discontinued in only 7% of patients due to an AE (26). This led to accelerated FDA approval for R/R MCL in 2013, making ibrutinib the most promising single agent for MCL at this time.
PI3K is another mediator in the BCR signaling pathway, and idelalisib is an oral inhibitor of the delta isoform of PI3K. A recent small (n=40) phase I study evaluated the efficacy of idelalisib in patients with heavily pretreated MCL. The ORR was 40% for the entire treatment population and 69% in those that received the chosen phase II dose of 150 mg BID or higher. The median DOR, however, was only 2.7 months, with a median PFS of 3.7 months. The high response rate, but low DOR, suggests that resistance to this drug is rapidly occurring. There was a small group of patients (22%) however that had durable responses lasting greater than a year. The major toxicities were transaminitis and diarrhea of any grade in 60% and 40% of patients respectively, with no significant myelosuppression or peripheral neuropathy noted (25). This study does provide rationale for targeting PI3K in MCL, and the unique toxicity profile makes it an ideal agent to study in combination other novel therapies mentioned above.
Future directions
The path going forward for MCL, in both the frontline and R/R settings, is promising. The incorporation of novel agents into standard chemoimmunotherapy combinations, along with maintenance treatment, is rapidly closing the gap between intensive and non-intensive frontline strategies allowing for high/durable responses with a more favorable toxicity profile, an important consideration in a disease that is predominantly of the elderly. Given the emergence of multiple novel agents for R/R disease, future studies should be directed at combining these agents in order to maximize treatment response/duration.
Acknowledgements
Disclosure: Saurabh Rajguru declares no conflict of interest; Brad S. Kahl: Consulting: Millennium, Roche/Genentech, Gilead, Teva, Celgene.
References
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997;89:3909-18. [PubMed]
- Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer 2008;113:791-8. [PubMed]
- Tiemann M, Schrader C, Klapper W, et al. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): a clinicopathological study from the European MCL Network. Br J Haematol 2005;131:29-38. [PubMed]
- Fernàndez V, Hartmann E, Ott G, et al. Pathogenesis of mantle-cell lymphoma: all oncogenic roads lead to dysregulation of cell cycle and DNA damage response pathways. J Clin Oncol 2005;23:6364-9. [PubMed]
- Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008;111:558-65. [PubMed]
- Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT). Blood 2010;115:1530-3. [PubMed]
- Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell 2003;3:185-97. [PubMed]
- Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol 2009;27:1209-13. [PubMed]
- Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood 2013;121:48-53. [PubMed]
- Hermine O, Hoster E, Szymczyk C, et al. Alternating Courses of 3x CHOP and 3x DHAP Plus Rituximab Followed by a High Dose ARA-C Containing Myeloablative Regimen and Autologous Stem Cell Transplantation (ASCT) Increases Overall Survival When Compared to 6 Courses of CHOP Plus Rituximab Followed by Myeloablative Radiochemotherapy and ASCT in Mantle Cell Lymphoma: Final Analysis of the MCL Younger Trial of the European Mantle Cell Lymphoma Network (MCL net). Available online: https://ash.confex.com/ash/2012/webprogram/Paper54717.html
- Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood 2008;112:2687-93. [PubMed]
- Geisler CH, Kolstad A, Laurell A, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol 2012;158:355-62. [PubMed]
- Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol 2005;23:7013-23. [PubMed]
- Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol 2010;150:200-8. [PubMed]
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012;367:520-31. [PubMed]
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [PubMed]
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944-52. [PubMed]
- Bernstein SH, Epner E, Unger JM, et al. A phase II multicenter trial of hyperCVAD MTX/Ara-C and rituximab in patients with previously untreated mantle cell lymphoma; SWOG 0213. Ann Oncol 2013;24:1587-93. [PubMed]
- Chang JE, Li H, Smith MR, et al. Phase 2 study of VcR-CVAD with maintenance rituximab for untreated mantle cell lymphoma: an Eastern Cooperative Oncology Group study (E1405). Blood 2014;123:1665-73. [PubMed]
- Ruan J, Martin P, Furman RR, et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol 2011;29:690-7. [PubMed]
- Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 2006;24:4867-74. [PubMed]
- Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol 2009;27:3822-9. [PubMed]
- Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 2013;31:3688-95. [PubMed]
- Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol 2012;13:716-23. [PubMed]
- Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3Kδ inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood 2014;123:3398-405. [PubMed]
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16. [PubMed]
- Wu L, Adams M, Carter T, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res 2008;14:4650-7. [PubMed]
- Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol 2009;84:553-9. [PubMed]


