
Breast cancer (BRCA) gene testing in ovarian cancer
Introduction
Ovarian cancer is the most lethal gynecological cancer of women with a 5-year survival rate of only 47% (1). This is mainly due to the fact that up to 59% of ovarian cancers are detected at advanced stages, for which survival is 29% (1). Cytoreductive surgery in combination with platinum- and taxane-based chemotherapy represents the gold standard for first-line therapy in ovarian cancer, and several studies have shown that gross residual disease correlates with survival (2). Two randomized phase III trials have been carried out adding bevacizumab to first-line chemotherapy and maintenance (3,4). Both studies suggested that the use of bevacizumab prolongs the median progression free survival in patients with advanced epithelial cancer. The consistency in the data across these trials, along with a prolific safety database, led to approval of bevacizumab for first-line maintenance in Europe and US (3,4). However, the recurrence rate in ovarian cancer is approximately 80%, even for patients who respond to initial treatment (5). Furthermore, it has been observed that recurring tumors have substantial heterogeneity due to multiple spontaneous genetic and epigenetic abnormalities. Represented clinically, this dynamic phenotype results in most patients undergoing multiple rounds of different chemotherapy, some with temporal benefit, but with nearly all patients succumbing to the emergence of drug resistance.
Both BRCA1 and BRCA2 are proteins involved in DNA double strand break repair by homologous recombination (HR). Loss of the functional fidelity of these proteins can lead to HR deficiency (HRD) and when present in the tumor, interventions inducing DNA double strand breaks can be differentially more lethal to the cancer cell. PARP, or poly-(ADP)-ribose polymerase, among other cellular processes, is instrumental in single strand DNA repair. When PARP is pharmacologically inhibited or trapped on DNA, single strand DNA breaks can become double strand breaks necessitating HR for repair. As is demonstrated below, conditions of HRD have provided clinical proof-of-concept for PARP inhibitor (PARPi) treatment (6). The rapidly expanding evidence-based for PARPi therapy in patients with ovarian and breast cancer has place a premium on identifying tumor-based HRD.
BRCA1 and BRCA2 germline and tissue mutations
Approximately 15% of ovarian cancer patients carry a germline mutation in BRCA1/2 (gBRCA1/2m) and approximately 7% of wild-type BRCA1/2 (wtBRCA1/2) ovarian cancer patients have a BRCA1/2 mutation in in their tumor (tBRCA1/2m) (7). These alterations are mostly found in high serous ovarian cancer, but have been reported in other histologies, including endometrioid carcinoma, clear cell carcinoma, low-grade serous carcinoma, and rarely carcinosarcomas (8). Since FDA approval of the first PARPi in 2014, ovarian cancer patients are routinely tested for gBRCA1/2m to determine their eligibility for PARPi therapy. Nevertheless, genetic testing of the tumor has shown that the presence of tBRCA1/2m may also predict PARPi efficacy (9). Thus, testing of tumor biopsies for tBRCA1/2m has gained traction and physicians now refer individuals to be tested for both germline and tumor-derived mutations. Findings from recent phase III clinical trials investigating PARPi (discussed below) in ovarian cancer patients confirm the validity in this approach (10-12).
Testing for BRCA1/2 mutations (BRCA1/2m) in ovarian cancer patients should also be accompanied by genetic counseling. Results from a prospective epidemiological study demonstrated that carriers of BRCA1m and BRCA2m have a cumulative risk of 60% and 55%, respectively for developing breast cancer and 59% and 17%, respectively, for developing ovarian cancer by 70 years (13). In families with a strong history of breast and ovarian cancer, individuals undergo genetic counseling and BRCA1/2 genotyping to identify germline mutation carriers. It is recommended that women with the mutations be screened regularly for early breast cancer detection and consider risk-reducing surgery. Furthermore, risk-reducing salpingo-oophorectomy is offered to patients between 35–40 years of age or after giving birth (14). Investigation into delayed oophorectomy following risk-reducing salpingectomy is underway as a potential option in delaying the adverse effects of premature ovarian loss in younger, at-risk women. Since family history alone underestimates the prevalence of BRCA1/2m among ovarian and breast cancer patients, current guidelines recommend testing all such patients regardless of family history (14). Therefore, risk assessment and screening initiation is extremely important in this risk population. Ideally, genetic counseling should occur prior to BRCA1/2m testing in order to discuss the implications of the positive results with the patient. However, limited availability of genetic counselors and potential delays in counseling services has shifted the paradigm to a “test now-counsel later” approach in some centers. In addition, while germline testing had preceded tumor testing or had been performed exclusive of tumor testing, recognition of the significant proportion of patients with BRCA1/2m present only in the tumor, has prompted several testing services to offer co-testing of both germline and tumor for mutations. How this information could be integrated into the patient management is presented in Figure 1.
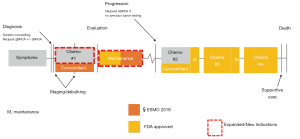
HRD in ovarian cancer
DNA lesions can be caused by exogenous or endogenous factors and can be comprised of either single-strand or double-strand breaks (Figure 2). During the single-strand break repair process, the base excision repair mechanism begins with the binding of the PARP complex to the DNA break, which further attracts DNA repair proteins like RAD51 to the site of damage (15). The high-fidelity DNA repair of the double-strand breaks by HR, occurs in the G2/M phase of the cell cycle and uses an available sister chromatid as a template. In the absence of the HR complex, DNA repair is performed by error-prone mechanisms such as non-homologous end joining (NHEJ) and alternate end joining (AEJ), which does not utilize a template and can lead to mutations, deletions, amplifications and chromosomal translocation, and eventually to cell death (16).
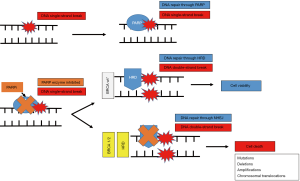
Although BRCA1/2 represents the most relevant clinical mediator of genomic instability that can be targeted therapeutically in ovarian cancer, the high proportion of tumors that share characteristics with BRCA1/2-deficient cancers, which don’t have the mutation itself, has established the new concept of BRCA1/2-like tumors. This term refers to the inability or reduced proficiency to perform HR due to the presence of other mutations or mechanisms outside of mutations in BRCA1/2, such as epigenetic hypermethylation of the BRCA1 promoter or loss-of-function mutations in other HR pathway genes (17). Norquist et al. examined the impact of HRD on clinical outcome by analyzing somatic and germline mutations in 14 HR genes using DNA from blood or tumor tissues from 1195 women. The results showed that a defective HR pathway correlates with significantly prolonged PFS and OS compared to patients without HR pathway mutations (18). Therefore, the positive response of patients with BRCA1/2m to platinum therapy may also apply to patients with HRD.
In addition to BRCA1/2, other genes involved in HR DNA repair include Fanconi anemia genes (PALB2, FANCA, FANCI, FANCL, FANCC), RAD genes (RAD50, RAD51, RAD51C, RAD54L), and DNA damage response (DDR) genes (ATM, ATR, CHEK1, CHEK2) (6). Alterations in other genes, such as PTEN homozygous loss or EMSY amplification, were identified as another potential mechanism of HRD (6). Molecular analyses of ovarian cancer performed using datasets from The Cancer Genome Atlas (TCGA) suggest that up to half of high grade serous ovarian cancers may have deficiencies in HR (7). Therefore, PARPi may be an effective therapy for a larger group of patients with HRD ovarian cancer in addition to patients with tumors carrying BRCA1/2m (19).
HRD testing is performed by DNA sequencing of tumor tissue, which can identify all types of mutations in key HR genes. The BROCA approach, a validated massively parallel sequencing assay, can be used to analyze a panel of 21 tumor suppressor genes, including BRCA1, BRCA2, and other genes known to be associated with HRD (20). This assay involves sequencing all exons, non-repeating introns, and select promoter regions of these genes in order to detect single-base substitutions, insertions, large deletions, duplications, and mosaicism. Another type of testing, the loss-of-heterozygosity (LOH) score was introduced as a quantitative marker of HRD in a phase 2 clinical trial, in which the primary endpoint was progression free survival after treatment with rucaparib in patients with wtBRCA1/2 with high or low LOH (21). PFS was significantly longer in the LOH high subgroup [HR of 0.62 (0.41–0.90), P=0.011] compared with the LOH low subgroup. The genomic LOH with a cutoff of 14% was assessed in archival and pretreatment biopsies using the Foundation Medicine T5 next-generation sequencing assay (Foundation Medicine, Cambridge, MA, USA) (21). The benefit in patients with a high genomic LOH and wtBRCA1/2 demonstrates the use of HRD as a predictive biomarker for sensitivity to PARPi. Another study proposed the tumor analysis of RAD51 foci as a functional biomarker of HR, which was able to show that increased RAD51 foci correlates with clinical response to PARPi (22,23). Recently, myChoice CDx (Myriad Genetics, Salt Lake City, UT, USA) became the first and only FDA approved tumor test that determines HRD status by detecting BRCA1 and BRCA2 (sequencing and large rearrangement) variants and assess genomic instability using three critical biomarkers: loss of heterozygosity, telomeric allelic imbalance and large-scale state transitions.
History of PARPi in clinical practice
Cloning of the BRCA1 and BRCA2 was achieved in 1994 and 1995, respectively (24,25). Furthermore, it was shown for the first time that the presence of BRCA1/2m influences patient susceptibility to breast and ovarian cancer (24,25). Nearly ten years later, two important preclinical studies established the potential importance of PARPi treatment for patients with BRCA1/2m (Figure 3).
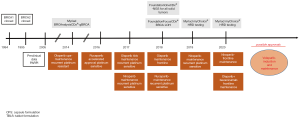
However, knowledge of the BRCA1/2 did not affect cancer treatment until 2009 when the first clinical studies reported improved clinical outcome in gBRCA1/2m carriers that received PARPi treatment (26,27). One of these trials by Fong and colleagues comprised of a phase I clinical trial (NCT00516373) to test the pharmacokinetic and pharmacodynamic characteristics of the first clinical-grade PARPi, olaparib. The study enrolled 60 patients with different solid tumors such as ovarian, breast, colorectal, melanoma, sarcoma, prostate, with a focus on gBRCA1/2m carriers. Overall, 19 patients, gBRCA1/2m carriers, were evaluated and 64% clinically benefited from treatment, which was assessed by imaging, tumor marker evaluation, or meaningful disease stabilization for a period of 4 months or more. Furthermore, the side effect profile was minimal, including grade 1–2 adverse events such as nausea (28%), vomiting (18%), anorexia (12%), dysgeusia (13%), or fatigue (28%). To extend upon this finding, the authors evaluated the effect of olaparib in 50 patients carrying a gBRCA1/2m with ovarian, primary peritoneal, or fallopian tube cancer, in correlation with platinum chemotherapy response (28). In this study, platinum sensitivity was evaluated among patients with clinical response to olaparib, which revealed 13 patients with platinum-sensitive disease, 24 with platinum-resistant disease and 13 with platinum-refractory disease (according to platinum-free interval). These findings suggest that platinum sensitivity in patients with gBRCA1/2m ovarian cancer may be associated with response to olaparib, with the greatest response in platinum sensitive patients, although substantial antitumor activity was still present in patients with platinum-resistant disease.
In light of the above-mentioned phase 1 and phase 2 monotherapy treatment studies, several phase 2 clinical trials investigating olaparib in previously treated patients either in combination with chemotherapy or as switch maintenance, defined by giving a new type of treatment after chemotherapy. Study 19 (NCT00753545) was among the first clinical trials evaluating the efficacy of olaparib monotherapy as switch maintenance treatment in patients with platinum-sensitive, relapsed high serous ovarian cancer who had partial or complete remission after their most recent dose of platinum-based chemotherapy (29). Platinum-sensitive patients were included due to clinical and in vitro data showing that cancer cells with defective HR are sensitized to PARPi after exposure to DNA damage inducing chemotherapy such as alkylating agents (30). Results of Study 19, a randomized, double-blind, placebo controlled, phase 2 trial, revealed an improvement in PFS from 4.8 to 8.6 months in patients treated with olaparib [HR of 0.35 (0.25–0.49) P<0.0001] and were presented in June 2014 for accelerated FDA approval. The study concluded that olaparib did not meet the favorable risk-benefit ratio criteria and therefore the accelerated approval request was rejected (31). Nevertheless, olaparib gained FDA approval together with the BRCAnalysis CDx test companion test (Myriad Genetics, Salt Lake City, UT, USA) for genomic BRCA1/2m in December 2014 based on efficacy data from Study 42 (NCT 01078662), a single-arm phase 2 study including 193 treated patients with platinum-resistant ovarian cancer who had a tumor response rate of 31% (95% CI, 24.6% to 38.1%) and stable disease (at >8 weeks) in 40% of patients (95% CI, 33.4% to 47.7%), confirming significant activity (32). SOLO2 (NCT01874353), a randomized, double-blind, placebo-controlled phase 3 trial that followed Study 19, enrolled only gBRCA1/2m platinum-sensitive patients and evaluated olaparib in switch maintenance for relapsed ovarian cancer patients who have received at least two lines of previous chemotherapy (27). These results showed that olaparib improved median progression free survival 19 vs. 5.5 months [HR of 0.3 (0.22–0.41) P<0001]. Based on the SOLO2 trial and the results from Study 19, the FDA extended the approval for olaparib in August 2017 for switch maintenance in platinum sensitive patients without consideration of BRCA1/2 mutation status. More recently survival analysis from SOLO2, demonstrated that maintenance with olaparib provided a clinically significant improvement of 12.9 months in median overall survival (33). The combination of olaparib with platinum/paclitaxel-based chemotherapy, studied by Oza and colleagues (NCT01081951), showed an improved PFS when compared to chemotherapy alone, with the greatest clinical benefit in BRCA1/2m patients [HR 0.21 (0.08–0.55)]. However, adverse events were reported at least 10% more frequently with olaparib plus chemotherapy than with chemotherapy alone, despite reduced chemotherapy and olaparib doses (34).
Although the above mentioned studies concluded with FDA approval of olaparib for patients with platinum-sensitive ovarian cancer regardless of BRCA1/2 mutation status, the reported efficacy of olaparib in patients with wtBRCA1/2 was still modest with a PFS of 7.4 vs. 5.5 months, showing PARPi over placebo effect [HR 0.54 (0.34–0.85) P=0.0075] (35). Therefore, the identification of a predictive biomarker for better selection of responders to PARPi therapy would be great benefit for this group of patients. This was the aim of ARIEL-2 (NCT01891344), a phase 2, open-label trial which showed that PFS was significantly increased in the BRCA1/2m [HR 0.27 (0.16–0.44) P<0.0001] and wtBRCA1/2 LOH high [HR 0.62 (0.42–0.90) P=0.011] compared to the wtBRCA1/2 LOH low patient group. Data from this study led to accelerated FDA approval for rucaparib and the companion LOH test, Foundation Medicine’s T5 NGS assay (Cambridge, MA, USA) in December 2016. To validate the findings from ARIEL-2, a second randomized, double-blind, placebo-controlled, phase 3 trial which enrolled 564 patients was initiated and observed a similar benefit in PFS, showing 10.8 vs. 5.4 months [HR 0.36 (0.30–0.45) P<0.0001] for patients with BRCA1/2m and wtBRCA1/2 with LOH high who received rucaparib. As a result, the FDA approved rucaparib in April 2018 for switch maintenance for recurrent platinum-sensitive ovarian cancer, regardless of molecular testing results.
Niraparib, was studied in a randomized, double/blind, phase 3 trial and showed anti-cancer activity in all patients with ovarian cancer. Furthermore, the enrolled patients were categorized according to the presence of a gBRCA1/2m, more exactly a gBRCA1/2m cohort and a non-gBRCA1/2m cohort including tBRCA1/2m, HRD and wtBRCA1/2 (12). The greatest median duration of progression-free survival was 21 vs. 5.5 months [HR 0.27 (0.17–0.41)] in the gBRCA1/2m cohort. Interestingly, niraparib treatment resulted in a longer PFS in both wtBRCA1/2 and HRD tumors, 12.9 vs. 3.8 months [HR 0.38 (0.24–0.59)]. In wtBRCA1/2 and HRP subgroups PFS was still increased with niraparib treatment, 9.3 vs. 3.9 months [HR 0.45 (0.34–0.61)]. Niraparib was approved by the FDA in March 2017 without any biomarker testing, setting precedent for the other two mentioned phase III switch maintenance trials. More recently in October 2019, FDA approved niraparib for the treatment of patients with advanced ovarian, fallopian tube, or primary peritoneal cancer who have been treated with >3 prior chemotherapy regimens, and whose cancer is associated with HRD status. This approval is based on results from QUADRA, the single-arm, phase 2 study, which showed that niraparib elicited an overall response rate (ORR) of 24% which was comprised of all partial responses, in the primary efficacy population (36). The FDA also approved in this setting the above mentioned HRD testing, myChoice CDx (Myriad Genetics, Salt Lake City, UT, USA) as a companion diagnostic to select patients for niraparib.
PARPi in first-line setting
After the emergence of the encouraging data using PARPi in recurrent tumors, several investigators evaluated the same therapy in patients with newly diagnosed advanced ovarian cancer, who are the only patients in whom treatment has curative potential. Initially, patients with newly diagnosed, advanced, platinum sensitive ovarian cancer with BRCA1/2m were enrolled in a randomized, double-blind, phase 3 trial which evaluated the efficacy of olaparib in switch maintenance (10). A total of 391 patients were randomized in this trial. Among these, 70% of the patients in the olaparib group had a lower risk of disease progression or death [HR 0.3 (0.23–0.41) P<0.001]. Furthermore, the estimated difference in median PFS between the olaparib group and the placebo group was approximately 3 years, a substantial benefit when compared to the 14-month PFS difference at the median in olaparib vs. placebo in recurrent tumors (37). Olaparib was approved by the FDA for the maintenance of ovarian cancer in BRCA1/2m carriers in December 2018.
Recently, a second PARPi given to platinum-sensitive patients in the switch maintenance after first-line therapy showed efficacy in HRD tumors and in the overall population. The PRIMA trial, a randomized, double-blind, phase 3 trial, enrolled 733 patients to receive niraparib or placebo. Patients with HRD tumors who received niraparib showed a significant longer median PFS of 21.9 vs. 10.4 months observed in patients receiving placebo [HR 0.43 (0.31–0.59) P<0.001]. The overall population treated with niraparib showed a similarly increased median PFS of 13.8 vs. 8.2 months [HR 0.70 (0.44–1.11)]. Interestingly, patients with HRP tumors benefited slightly from niraparib treatment, showing a median PFS of 8.1 vs. the 5.4 months observed in the placebo group (HR 0.68) (38).
While SOLO1 enrolled only patients with BRCA1/2m, which was the patient population that exhibited the greatest benefit from PARPi therapy, the PRIMA trial investigated the effect of niraparib in the overall population and in patients with HRD and wtBRCA1/2. Results from the PRIMA trial aligned with the data from the NOVA trial, specifically the greatest increase in PFS in HRD patients.
Bevacizumab is an important drug used for maintenance therapy in primary tumors for patients with advanced disease. Bevacizumab is an anti-angiogenic monoclonal antibody, neutralizing the VEGF ligand, which gained approval in Europe in June 2014 and June 2018 in the United States based on GOG-218 and ICON7 findings (3,4). GOG-218 was a randomized, double-blind, placebo-controlled phase 3 trial in which 1,873 patients with stage III or IV ovarian cancer who underwent debulking surgery were assigned to one of the three arms of the study, each of which included a carboplatin-paclitaxel based chemotherapy: placebo given in cycles 2 through 22, bevacizumab-initiation treatment given in cycles 2 through 6 then placebo in cycles 7 through 22, and bevacizumab given in cycles 2 through 22. The median PFS was 10.3 months in the placebo group, 11.2 in the bevacizumab-initiation group [HR 0.91 (0.8–1.04)], and 14.1 months in the bevacizumab-throughout group [HR 0.72 (0.63–0.82)] (3). ICON 7 was a phase 3 clinical trial in which 1,528 patients with similar disease characteristics as those in GOG-218 were enrolled and randomly assigned to one of two treatment regimens: carboplatin-paclitaxel standard of care alone or in combination with bevacizumab given in cycles 2 through 22. After 36 months, PFS was 20.3 months with standard therapy vs. 21.8 months with addition of bevacizumab [HR 0.81 (0.70–0.94) P<0.0004] (4).
FDA approvals for first-line maintenance monotherapy started with bevacizumab approval in patients with newly diagnosed advanced ovarian cancer, regardless of their BRCA1/2 mutation status (3,4). Similarly, olaparib showed substantial PFS benefit as first-line maintenance monotherapy for patients with BRCA1/2m and was approved by the FDA in December 2018 (10). Therefore, assessment of PFS after combining the two agents for the treatment of primary tumors was the aim of PAOLA-1, a phase 3 trial which analyzed PARPi for maintenance therapy in patients with advanced ovarian cancer regardless of BRCA1/2 mutation status receiving first-line standard of care treatment (39). Median PFS increased in patients receiving olaparib plus bevacizumab compared to placebo plus bevacizumab, which was 22.1 vs. 16.6 months, respectively [HR 0.59 (0.49–0.72) P<0.0001]. As expected, the longest PFS was observed in patients with BRCA1/2m treated with olaparib plus bevacizumab, whose median PFS was 37.2 months [HR 0.31 (0.20–0.47)] compared to 21.7 months of wtBRCA1/2 patients [HR 0.71 (0.58–0.88)]. Furthermore, analysis of patients in the olaparib plus bevacizumab study arm with HRD revealed a longer PFS in patients with HRD tumors, especially HRD with BRCA1/2m, which was 37.2 vs. 17.7 months [HR 0.33 (0.25–0.45)], and the PFS of HRD excluding BRCA1/2m was 28.1 vs. 16.6 months [HR 0.43 (0.28–0.66)]. There was no improvement of PFS in HRP patients. Based on these findings, FDA approved olaparib plus bevacizumab as maintenance for ovarian cancer patients in May 2020.
PAOLA-1 was the first study to combine two targeted therapies in primary tumor maintenance and to use an active control arm. The combination of PARPi with full-dose chemotherapy has been a challenge due to the toxicity of the combination (34). Nevertheless, the induction DNA damage by chemotherapy may enhance the efficacy of PARPi due to the increased requirement of the cell for DNA repair. Based on this rationale, Coleman and colleagues reported the results of a randomized phase 3, placebo-controlled trial which assessed the efficacy of a new PARPi, veliparib, added to first-line full-dose chemotherapy including carboplatin and paclitaxel and continued as maintenance monotherapy (40). A total of 1,140 patients with previously untreated stage III or IV high-grade serous ovarian cancer who underwent cytoreductive surgery before initiation or after 3 cycles were randomized to one of the three study arms: chemotherapy plus placebo followed by placebo maintenance, chemotherapy with veliparib followed by placebo maintenance, or chemotherapy plus veliparib followed by veliparib maintenance. Interestingly, because the main objective of this study was to test whether concurrent therapy with veliparib, with or without veliparib maintenance therapy, could improve progression-free survival, this study did not include a veliparib maintenance-only group in contrast to other studies. The enrolled patients were divided into different cohorts depending on their BRCA1/2 mutation status. Patients were randomized at diagnosis and thus were analyzed for PFS (primary endpoint) from that point and included all patients regardless of response to induction therapy. The trial also allowed for neoadjuvant chemotherapy and dose-dense paclitaxel, representing commonly used approaches in primary ovarian cancer management. This study concluded that patients treated with veliparib throughout exhibited significantly increased PFS compared to patients receiving chemotherapy alone. The greatest PFS benefit for the veliparib throughout arm was observed in the patients with ovarian BRCA1/2m, 34.7 vs. 22 months [HR 0.44 (0.28–0.68) P<0.001]. Further, in tumors with HRD (including those with BRCA1/2m) the median PFS was 31.9 months compared to 20.5 months [HR 0.57 (0.43–0.76) P<0.001]. The median PFS in the intention-to-treat population was 23.5 vs. 17.3 months [HR 0.68 (0.56–0.83) P<0.001].
The results of the recently published studies with proven efficacy of PARPi have provided several treatment options for PARPi monotherapies or in combination with other classes of agents for ovarian cancer patients after primary diagnosis. While the SOLO1 trial was limited to olaparib monotherapy in switch maintenance, the PAOLA-1 trial investigated the combination of olaparib and bevacizumab because most patients with advanced disease have already received bevacizumab during induction therapy. The addition of olaparib to bevacizumab maintenance resulted in a significant increase in PFS of patients with ovarian cancer BRCA1/2m and HRD. Nevertheless, considering the increase in PFS of patients treated with PARPi during induction chemotherapy and maintenance in the VELIA trial, integrating BRCA1/2m, and possibly HRD testing, into therapy management as early as possible after first diagnosis. We present these findings and rationale for first-line therapy management of patients with advanced ovarian cancer by comparing algorithms based on FDA approvals 2019 and the more recently FDA approvals 2020 (Figure 4A,B). Our models emphasize the paradigm shift for first-line therapy with PARPi and predict how these findings will reshape the treatment landscape of advanced ovarian cancer.
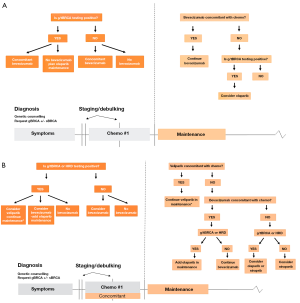
Clinical challenges of including PARPi in all settings
Fortunately, due to recent advances with PARPi in first-line setting, an increasing number of patients will likely receive this therapy earlier in their standard treatment course. Therefore, concerns about need to identify functional biomarkers to better predict PARPi sensitivity, emerging of tumor resistance, prior exposure to PARPi, and how these affect the outcome of subsequent therapeutic regimens represent new challenges. Thus, enabling more personalized care through the development of tools to help oncologists identify tumors that harbor HR suppression and hypersensitivity to specific classes of chemotherapeutic agents will inform therapy selection in individual patients (41).
A key mechanism of resistance for BRCA1/2-mutated cancers to PARPi is the acquisition of BRCA1/2 reversion mutations that restore the wild-type sequence, leading to the expression of functional protein (42). A recent study investigated the presence of BRCA1/2 reversion mutations in clinical samples which were collected from BRCA1/2m carriers before treatment with rucaparib and tumors post-progression (43). A total of 78 samples were analyzed using next-generation sequencing of circulating cell-free DNA extracted from plasma, which revealed that rucaparib and platinum resistance correlates with BRCA1/2 reversion. The PFS of BRCA1/2m carriers without reversion mutations was significantly longer than that of BRCA1/2m carriers with reversion mutations [HR 0.12 (0.05–0.26) P<0.0001]. Presence of the reversion mutation may predict response to therapy, though there is no established clinical routine, and there are notable examples where PARPi resistance does not implicate platinum-resistance. Therefore, emergence of PARPi resistance through hyperactivation of NHEJ function represents a concept that does not apply in the case of platinum agents which is NHEJ independent (44). Some have reported reversion mutations in several homologous repair pathway genes other than BRCA1/2, such as RAD51C and RAD51D (45). Other potential mechanisms of acquired resistance to PARPi reported by preclinical studies on BRCA1m carcinomas include modulation of TP53 binding protein 1 (53BP1), which maintains the balance between HR and NHEJ. More precisely the 53BP1 knock-out efficiently rescued HR and induced PARPi resistance (46). However, the only clinically validated mechanism is the acquisition of BRCA1/2 reversion mutations (47).
Future directions in the PARPi field will be to increase PARP inhibition efficiency though combination therapies with different classes of agents such as immune checkpoint inhibitors, anti-angiogenic agents, or other targeted agents, such as those involved with HR or DNA division checkpoints. Treatment with olaparib in BRCA1-deficient mice showed an increase in CD4+ and CD8+ cells and a significant increase in their production of IFN-gamma and TNF-alpha (48). Interestingly, the increase in CD4+ and CD8+ led to the recruitment of dendritic cells, which are potent antigen presenting cells. In the same study, expression of PD-L1 was increased, which led to the hypothesis that the addition of PD-1 immune checkpoint blockade would prolong PARP inhibition by overcoming the increased expression of PD-L1 on tumor cells. There are several clinical trials which evaluate the combination between PARPi and immune checkpoint inhibitors for first-line therapy. The ATHENA trial (NCT 03522246), a phase 3, randomized, multinational 4-arm study evaluating the response of patients to rucaparib and nivolumab (anti-PD-1) as maintenance treatment following first-line treatment. The FIRST trial (NCT 03602859), a phase 3 randomized trial is a European 2-arm study which compares the combination of platinum and a PD-L1 inhibitor followed by niraparib and PD-L1 inhibitor maintenance vs. standard of care platinum-based treatment in patients with stage III or IV ovarian cancer. The DUO-O trial (NCT 03737643) is another phase 3, randomized, multi-centric trial which evaluates durvalumab in combination with standard of care platinum-based chemotherapy and bevacizumab followed by maintenance with both durvalumab and bevacizumab or durvalumab, bevacizumab and olaparib in patients with newly diagnosed ovarian cancer. A fourth clinical trial is ENGOT-ov43 (NCT 03740165) which studies chemotherapy with pembrolizumab followed by maintenance with olaparib. Most of the above-mentioned trials are estimated to be completed between 2023 and 2025.
Another preclinical study investigating the combination of PARPi with cediranib, an antiangiogenic agent, revealed promising drug to drug interactions. The proposed mechanism of action of cediranib is the induction of hypoxia by activating PP2A and suppressing gene expression through the E2F4/p130 complex, triggering the upregulation of HR repair. This leads to increased tumor sensitivity to PARPi (49). Several phase 3 clinical trials are ongoing to evaluate the combination of olaparib and cediranib, regardless of patient BRCA1/2 mutation status, such as ICON9 (NCT03278717), NRG-GY004 (NCT02446600), and NRGGY005 (NCT02502266) which will be completed in 2023–2024.
Due to the data from preclinical studies supporting the existing hypothesis that targeting other cancer promoting pathway components such as WEE1, MEK/MAPK, and PI3K will enhance the efficacy of PARPi, there is increasing interest in the combination of PARPi with other targeted agents. WEE1 inhibition induces replication-dependent DNA damage due to aberrant DNA replication through CDK2, a cyclin-dependent kinase which plays a critical role in the activation of the G2/M (50). Interestingly, another study revealed an increase in the RAS/mitogen activated MAPK pathway in PARP resistant cells, which was reversed by MEK or ERK inhibitors (51). Nevertheless, the synergy between PI3K and PARP inhibitors is driven by the antimetabolic activity of PI3K inhibitors, which lowers available nucleotides required for DNA synthesis and S-phase progression (52). Several clinical trials using different drug combinations to overcome PARPi resistance are under way. EFFORT (NCT03579316) is a randomized, phase 2 trial with two arms, olaparib monotherapy and olaparib combined with a WEE1 inhibitor, in patients who have progressed on PARPi therapy. The primary endpoint of this study is to evaluate objective response or partial response, while the secondary endpoint is to evaluate the extent of disease control. This trial began enrolling patients in December 2018 and has an estimated completion date of October 2020. A second trial, SOLAR (NCT03162627), for patients who have developed PARP resistance, is a randomized phase I and II trial combining olaparib with the MEK/MAPK and ERK inhibitor selumetinib. This study began in August 2017 and has an estimated completion date of August 2026. Furthermore, a phase Ib trial, which is expected to have results in 2022, investigated the optimal dose and side effects of niraparib and the PI3K inhibitor copanilisib in patients with relapsed endometrial, ovarian, primary peritoneal, or fallopian tube cancer. BRCA1/2 mutation status is not a criterion for patient enrollment in any of the above-mentioned trials.
Conclusions and future directions
Along with the development of PARPi in relapsed and primary tumors, BRCA1/2m were established as a biomarker, initially used for patient selection to PARPi therapy. Due to the data from the ARIEL-3, PRIMA, NOVA, VELIA, and PAOLA-1 trials among others revealing a clinical benefit from patient stratification based on biomarkers such as HRD and genomic LOH, the recommendation for PARPi therapy has extended from BRCA1/2m carriers to a greater population of ovarian cancer patients. While BRCA1/2m influence patient prognosis and patients with these mutations have improved response to platinum therapy and PARPi, the BRCA1/2m biomarker is no longer used alone for selection of patients for PARPi therapy. Therefore, BRCA1/2m and possibly HRD testing should be performed as early as possible in the course of treatment. Similarly, the PAOLA-1 trial revealed that patients with BRCA1/2m or HRD benefit from a combination of olaparib and bevacizumab as first-line therapy. This is especially important considering that most of the patients with advanced disease will have already been treated with bevacizumab, which offers them the opportunity to add olaparib to the preexisting maintenance regimen. Nevertheless, the PRIMA trial also showed that patients with HRD benefit from the single agent niraparib, which is in agreement with the other trials and emphasizes the importance of BRCA1/2m and HRD testing in deciding best options for first-line therapy management (Figure 4A,B).
Current data with PARPi provides a framework for future studies to further evaluate DNA damage repair as a potential cancer vulnerability and to improve PARPi in cancer treatment. The greatest challenge of this treatment option is identification of patients who are ideal candidates for single agent vs. combination, in the light of prior exposure or resistance to therapy.
Acknowledgments
Editorial support was provided by Amanda R. Haltom.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Shaheenah Dawood) for the series “Targeting the DNA Damaging Pathway: PARPi and Beyond” published in Chinese Clinical Oncology. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco-20-4). The series “Targeting the DNA Damaging Pathway: PARPi and Beyond” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society- Cancer Facts and Figures 2019 [database on the Internet]. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer 2009;115:1234-44. [Crossref] [PubMed]
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011;365:2473-83. [Crossref] [PubMed]
- Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484-96. [Crossref] [PubMed]
- Monk BJ, Herzog TJ, Tewari KS. Evolution of Chemosensitivity and Resistance Assays as Predictors of Clinical Outcomes in Epithelial Ovarian Cancer Patients. Curr Pharm Des 2016;22:4717-28. [Crossref] [PubMed]
- Konstantinopoulos PA, Ceccaldi R, Shapiro GI, et al. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov 2015;5:1137-54. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609-15. [Crossref] [PubMed]
- Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol 2012;30:2654-63. [Crossref] [PubMed]
- Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol 2010;28:3570-6. [Crossref] [PubMed]
- Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2018;379:2495-505. [Crossref] [PubMed]
- Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:1949-61. [Crossref] [PubMed]
- Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 2016;375:2154-64. [Crossref] [PubMed]
- Mavaddat N, Peock S, Frost D, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 2013;105:812-22. [Crossref] [PubMed]
- NCCN Guidelines 2019 Version 3.2019.
- Noordermeer SM, van Attikum H. PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells. Trends Cell Biol 2019;29:820-34. [Crossref] [PubMed]
- Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 2009;9:415-28. [Crossref] [PubMed]
- Konstantinopoulos PA, Spentzos D, Karlan BY, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol 2010;28:3555-61. [Crossref] [PubMed]
- Norquist BM, Brady MF, Harrell MI, et al. Mutations in Homologous Recombination Genes and Outcomes in Ovarian Carcinoma Patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res 2018;24:777-83. [Crossref] [PubMed]
- Moschetta M, George A, Kaye SB, et al. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol 2016;27:1449-55. [Crossref] [PubMed]
- Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A 2011;108:18032-7. [Crossref] [PubMed]
- Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 2017;18:75-87. [Crossref] [PubMed]
- Cruz C, Castroviejo-Bermejo M, Gutierrez-Enriquez S, et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol 2018;29:1203-10. [Crossref] [PubMed]
- Castroviejo-Bermejo M, Cruz C, Llop-Guevara A, et al. A RAD51 assay feasible in routine tumor samples calls PARP inhibitor response beyond BRCA mutation. EMBO Mol Med 2018;10:e9172. [Crossref] [PubMed]
- Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994;266:66-71. [Crossref] [PubMed]
- Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995;378:789-92. [Crossref] [PubMed]
- Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123-34. [Crossref] [PubMed]
- Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010;376:245-51. [Crossref] [PubMed]
- Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 2010;28:2512-9. [Crossref] [PubMed]
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012;366:1382-92. [Crossref] [PubMed]
- Taniguchi T, Tischkowitz M, Ameziane N, et al. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med 2003;9:568-74. [Crossref] [PubMed]
- Kim G, Ison G, McKee AE, et al. FDA Approval Summary: Olaparib Monotherapy in Patients with Deleterious Germline BRCA-Mutated Advanced Ovarian Cancer Treated with Three or More Lines of Chemotherapy. Clin Cancer Res 2015;21:4257-61. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Poveda A. Final overall survival (OS) results from SOLO2/ENGOT-ov21: A phase III trial assessing maintenance olaparib in patients with platinum-sensitive, relapsed ovarian cancer and BRCA mutation. ASCO 2020.
- Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 2015;16:87-97. [Crossref] [PubMed]
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014;15:852-61. [Crossref] [PubMed]
- Moore KN, Secord AA, Geller MA, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2019;20:636-48. [Crossref] [PubMed]
- Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1274-84. [Crossref] [PubMed]
- Gonzalez-Martin A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2019;381:2391-402. [Crossref] [PubMed]
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med 2019;381:2416-28. [Crossref] [PubMed]
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med 2019;381:2403-15. [Crossref] [PubMed]
- Pitroda SP, Pashtan IM, Logan HL, et al. DNA repair pathway gene expression score correlates with repair proficiency and tumor sensitivity to chemotherapy. Sci Transl Med 2014;6:229ra42. [Crossref] [PubMed]
- Domchek SM. Reversion Mutations with Clinical Use of PARP Inhibitors: Many Genes, Many Versions. Cancer Discov 2017;7:937-9. [Crossref] [PubMed]
- Lin KK, Harrell MI, Oza AM, et al. BRCA Reversion Mutations in Circulating Tumor DNA Predict Primary and Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov 2019;9:210-9. [Crossref] [PubMed]
- McCormick A, Donoghue P, Dixon M, et al. Ovarian Cancers Harbor Defects in Nonhomologous End Joining Resulting in Resistance to Rucaparib. Clin Cancer Res 2017;23:2050-60. [Crossref] [PubMed]
- Goodall J, Mateo J, Yuan W, et al. Circulating Cell-Free DNA to Guide Prostate Cancer Treatment with PARP Inhibition. Cancer Discov 2017;7:1006-17. [Crossref] [PubMed]
- Nacson J, Krais JJ, Bernhardy AJ, et al. BRCA1 Mutation-Specific Responses to 53BP1 Loss-Induced Homologous Recombination and PARP Inhibitor Resistance. Cell Rep 2018;24:3513-27.e7. [Crossref] [PubMed]
- Lord CJ, Ashworth A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med 2013;19:1381-8. [Crossref] [PubMed]
- Ding L, Kim HJ, Wang Q, et al. PARP Inhibition Elicits STING-Dependent Antitumor Immunity in Brca1-Deficient Ovarian Cancer. Cell Rep 2018;25:2972-80.e5. [Crossref] [PubMed]
- Kaplan AR, Gueble SE, Liu Y, et al. Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD51. Sci Transl Med 2019;11:eaav4508.
- Guertin AD, Li J, Liu Y, et al. Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy. Mol Cancer Ther 2013;12:1442-52. [Crossref] [PubMed]
- Sun C, Fang Y, Yin J, et al. Rational combination therapy with PARP and MEK inhibitors capitalizes on therapeutic liabilities in RAS mutant cancers. Sci Transl Med 2017;9:eaal5148.
- Hu H, Juvekar A, Lyssiotis CA, et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell 2016;164:433-46. [Crossref] [PubMed]


