Clinical trials in lung cancer surgery and research cooperation
Cooperative groups
In the United States, there are four general oncology cooperative groups active in lung cancer researches: the Eastern Cooperative Oncology Group (ECOG), Southwest Oncology Group (SWOG), Cancer and Leukemia Group B (CALGB) and the North Central Cancer Treatment Group (NCCTG). The ECOG, SWOG, and CALGB include member institutions throughout the whole country, while NCCTG is a regional cooperative group centered at the Mayo Clinic. In Canada, the National Cancer Institute-Canadian Clinical Trials Group (NCIC CTG) oversees cooperative oncology efforts (1-3). In addition, two more focused cooperative oncology groups who play a pivotal role and work across the US/Canadian border are the American College of Surgeons Oncology Group (ACOSOG) (4) and the Radiation Therapy Oncology Group (RTOG). There are multiple cooperative groups in Europe, mostly based on countries, but the EORTC spans multiple countries and has been a major contributor to critical trials in lung cancer. The Japan Clinical Oncology Group (JCOG) is a cooperative oncology group with the aim of conducting, developing, coordinating and stimulating clinical researches in Japan targeting at treatments of cancers and related problems (5).
Clinical trials in lung cancer surgery
Clinical research in non-small-cell lung cancer (NSCLC) is a rapidly evolving field. In an effort to identify current trends in lung cancer clinical researches, Subramanian et al. reviewed ongoing clinical trials in NSCLC registered on ClinicalTrials.gov in 2012. Comparing the data with a similar survey conducted in 2009, they figured out a significant increase in trials conducted exclusively outside the United States (35.9-48.8%; P=0.001). The number of studies originated from China (61, 12.8%) was second only to that in the United States (244, 51.2%). The majority of 477 trials included were multicenter studies (56.8%) and a large proportion of phase II and III clinical trials (77.2%) were focused on patients with advanced-stage diseases. What’s more, results indicated that there were three times more studies performing biomarker testing to determine how to select patients than 3 years ago (6).
We used the advanced search option available on the ClinicalTrials.gov to review clinical trials regarding surgical issues. In the drop down menus, we chose “all studies” for recruitment status and “interventional” for study type and all trials reported until Aug, 2013 on the website were included. We extracted the following information: (I) type of clinical trial (phase I, II, or III); (II) recruiting status; (III) study design: randomization, control group, and number of study arms; (IV) location; (V) the number of trial centers; (VI) primary sponsors; (VII) treatment setting; (VIII) date of trial activation; (IX) time completed since the study was open for enrollment; and (X) primary outcome measure. We excluded trials that did not involve patients with lung cancer surgery and that did not include any form of medical therapies. Finally, 658 studies found for: surgery or adjuvant or neo-adjuvant—interventional studies—lung cancer. Lung cancer surgical clinical trials mainly include trials related to the procedure (92 records, 13.9%) and (neo)adjuvant therapy (370 records, 56.2%). Phase III trials account for 15.5%. Only 34.9% trials were completed, among which 43 studies presented results.
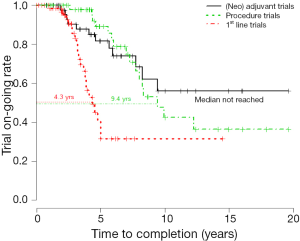
Furthermore, phase III trials with lung cancer procedure (56 records), (neo)adjuvant therapy (15 records in neo-adjuvant and 34 records in adjuvant) and first line setting (105 records) were analyzed respectively, and the median time to completion (MTC) between groups were calculated in Kaplan-Meier curve in software R. The completed trials were regarded as completed data, other trials (active, not recruiting; not yet recruiting; recruiting; suspended; withdrawn) as censored data. The MTC of procedure trials in lung cancer surgery is 9.4 years. The MTC in neo-adjuvant and adjuvant trials have not been reached but longer than 10 years. However, the MTC in first line setting is only 4.3 years (Figure 1).
Clinical trials in neo-adjuvant therapy are characterized as having difficulties of enrollment and completion rate. In 14 phase III studies of neo-adjuvant therapy, only 2 trials were completed. CALGB 9734 (combination radiation with or without chemotherapy therapy in treating patients with stage IIIA non-small cell lung cancer) set up the initial accrual goal of 480 patients, but was closed ahead of schedule due to slow accrual just two years later since activated. The CHEST trial attempted to assess benefits from three cycles of preoperative cisplatin/gemcitabine versus surgeries alone (7). Designed to enroll 712 patients with stage IB-IIIA NSCLC, this trial attempted to detect an improvement of 20% in PFS as the primary endpoint. However, from 2000 to 2004, only 270 patients were enrolled at 45 centers in 15 countries throughout Europe. It prematurely closed in light of mounting evidences supporting a role for chemotherapy. Hereafter, two other RCTs were directly evaluating the efficacy of neoadjuvant and adjuvant chemotherapy. The three-arm Neoadjuvant/Adjuvant Taxel/Carboplatin Hope (NATCH) trial accrued 624 patients with surgery alone versus three cycles of preoperative or postoperative paclitaxel/carboplatin chemotherapy plus surgery in early-stage NSCLC from 1999 to 2011, with the comparison of disease-free survival (DFS) (8). Then, Chinese Society of Lung Cancer (CSLC) initiated a head-to-head RCT comparing neoadjuvant with adjuvant cisplatin/docetaxel (CSLC 0501, NCT00321334) in patients with stage I-IIIA lung cancer in 2006. The planned sample size was 410 over 4 years. From March 2006 to May 2011, 198 patients had been accrued from 20 centers and the trial was shut off in May 2011 owing to poor accrual. CSLC 0501 indicated that neoadjuvant or adjuvant chemotherapy with docetaxel plus carboplatin in resectable clinical stage IB-IIIA NSCLC is feasible and safe. The 3-year DFS was 45% in the neoadjuvant arm and 53% in the adjuvant arm (HR =0.88; 95% CI, 0.58-1.33; P=0.54). Median survival has not been reached in both arms.
Clinical trials in adjuvant therapy are characterized with better enrollment but a long-lasting follow-up time. Compared with a neo-adjuvant chemotherapy strategy, adjuvant therapy is more appealing to thoracic surgeons and most patients. Since surgeons can appoint a certain time for surgeries at patients’ initial visit instead of sending them to medical oncologists and waiting for 3 months, patients will get rid of probabilities of progression and experiencing side effects which keep them from undergoing surgeries. International Adjuvant Lung Cancer Trial (IALT), which proved positive effects of adjuvant cisplatin-based combination chemotherapy on resectable stage I-III NSCLC with 4.7 years of follow-up, demonstrated negative results after 7.5 years of follow-up and increasing risks of non-cancer deaths in adjuvant group (9). The BR19 trial reporting adjuvant gefitinib after complete resection of early stage NSCLC (stage IB 49%, II 38%, III 13%), failed to confer DFS or overall survival (OS) advantages in overall population (10). The poor results should be firstly blamed on a short time of only 4.8 months as median gefitinib treatment time. Secondly, stage IB lung cancers accounted for nearly half of enrolled patients, which gained no benefits from the transitional adjuvant chemotherapy. Besides, there were only 76 patients with epidermal growth factor receptor (EGFR) mutations in this analysis, degrading the strength of evidences. BR19 closed prematurely in 2005 after ISEL trial provided negative conclusions. Shortly thereafter, SWOG S0023 study indicated fruitless results and even proved potentially harmful effects with gefitinib. Bio-marker driven trials in selected lymph nodes positive population should be carried out in adjuvant settings based on tumor biological characteristics and/or individual heterogeneity of sensitivity to agents. Such researches are performed on foundations of surgical specimens, emphasizing the significant value of surgeons acting as investigators, coordinators and cooperators at the same time.
It has been denounced for years that randomized controlled trials (RCT) in surgery are in default. What’s worse, there exist some factual and inevitable obstacles of implementing evidence-based medicine into surgeries. Although randomized controlled studies in internal medicine were originally developed to compare and estimate two different drug protocols, there are some intrinsic pitfalls and bias in surgical randomized trials. Furthermore, it remains insurmountable to standardize tested surgical procedures due to different institutions, different surgeons and even different surgeries. Efficacy of surgeries lays more dependence on surgeons’ increasing experiences. With surgeons conquering the learning curve, complications along with surgeries diminish, and patients benefit more from new procedures (11). In CALGB 39802 (12), only surgeons who have performed at least five VATS lobectomy before being credentialed were qualified to participate and were required to undergo a rigorous credentialing protocol that included registration in a course to review techniques, submission of an unedited videotape, operative and pathological reports from a VATS lobectomy for central review, and participation in an animal laboratory. However, through such overly restrictive exclusion criteria, the group of patients eligible for enrollment in a RCT may comprise an unrepresentative part of typical patients. Patients’ admission to trials is tough and subsequential follow-up is lengthy and exhausting. In addition, financial supports for surgical clinical researches are limited and there seems to be a lack of interest in funding of surgical trials.
Given the circumstances, ACOSOG Z0030 (Study Chair Dr. Mark Allen) is a phase III trial comparing mediastinal lymphadenectomy with mediastinal lymph node sampling for T1N0/non-hilar N1 NSCLC. A total of 1,000 patients (500 per arm) were planned to be accrued within 5 years. This trial enrolled 1,111 patients in less than 5 years. To ensure representativeness of targeted patients, disease characteristics related to N status include: biopsy proven or suspected; clinically resectable stage I-II (T1 or T2, N0 or non-hilar N1, M0) NSCLC; if preoperative mediastinoscopy has been performed, hilar lymph nodes must be certified to be less than 1 cm in the short axis and no N2 disease is observed on preoperative CT scan; if preoperative mediastinoscopy has not been performed, all lymph nodes of hilum and mediastinum must be measured less than 1 cm in the short axis on preoperative CT scan. Darling GE and colleagues recently demonstrated no difference in survival between these two procedures (13).
Clinical trials comparing surgical results between lobectomy and sublobar resection (segmentectomy or wedge resection) for clinical T1aN0M0 NSCLC are currently being conducted by Cancer and Leukemia Group B (CALGB 140503) and the Japan Clinical Oncology Group/West Japan Oncology Group (JCOG0802/WJOG4607L). Their results would indicate the significance of sublobar resection for early NSCLC. Cancer and Leukemia Group B (CALGB) has activated a phase III randomized trial (CALGB 140503) of lobectomy versus sublobar resection for ≤2 cm, node-negative and peripheral NSCLC, that is, tumors with its center located in the outer third of the lung field. Patients are randomized intraoperatively into lobectomy or sublobar resection following confirmation of N0 status by frozen section of mediastinal and major hilar nodal stations. The primary end point is DFS and secondary end points include OS, rate of locoregional and systemic recurrence, and pulmonary function measured by pulmonary expiratory flow rate 6 months after the surgery. The target accrual is 908 randomized patients over 5 years with 3 years of follow-up. The trial is supported by most American cooperative groups as well as National Cancer Institute of Canada. Concurrently, a multi-institutional trial is initiated by Japan Clinical Oncology Group (JCOG0802) The trial will enroll patients with 2 cm or less peripheral adenocarcinoma and a component of less than 25% GGO and randomly assign patients to segmentectomy or lobectomy. The initial target is to recruit 1,100 cases within 3 years followed by 5 years of follow-up. Currently on June, 2013, 538 cases have been enrolled. Although enrollments of these two trials are slower than expected, it is of vast probability for our textbook to be revised based on their results (14).
Chinese Thoracic Oncology Group (CTONG)
The number of lung cancer patients in China is increasingly accelerating by up to 500,000 annually, which should be blamed on the largest quantities of tobacco products and smokers. So China would play a leading role in fields of lung cancer researches as long as with effective combinations of sponsorship from pharmaceutical manufacturers, experienced researchers and abundant resources of patients. From 2001-2006, several small-scale multicenter RCT for adjuvant and neo-adjuvant therapy were established by the organization of Chinese society of lung cancer. Meanwhile, with arrival of a new epoch based on individual targeted therapy, lung cancer researchers in China began to participate in the most important international clinical trials, such as INTETEST, IPASS, FASTACT, JMEN, TRUST, and so on, have access to professional training, contribute a large amount of lung cancer cases, and make Chinese voice heard. In 2007, based on international trial experiences, a national collaborative clinical research group, CTONG, was established. CTONG is a large network of researchers, surgeons, medical oncologists, radiation oncologists, statisticians, nurses/clinical research associates, health-care professionals and patient advocates at public institutions throughout China. Up to now, there have already been 25 top member hospitals (15). The purpose of this collaborative group is to carry out multi-center clinical trials for lung cancer, and provide high level of evidence-based medicine for clinical practices and translational researches. CTONG, an extremely active and well-organized study group, takes responsibility of carrying out phase I-IV clinical trials and translational research, establishing database of lung cancer patients and tissue bank, and promoting professional trainings as well as international communications. More than 10,000 new cases of lung cancer have been diagnosed and treated in CTONG hospitals every year, thus enabling China to initiate bio-marker driven trials rapidly. CTONG have already published major studies such as OPTIMAL, INFORM and FASTACT 2 just within a few years since establishment, contributing substantially to lung cancer patients and being expected to obtain more triumphs.
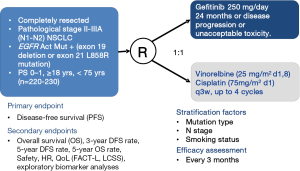
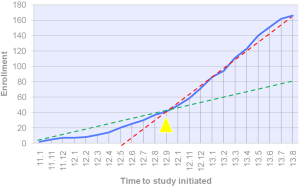
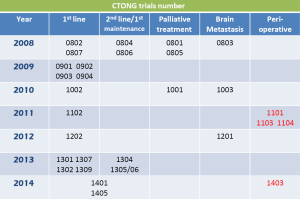
Apart from trials for advanced NSCLC, series of peri-operative trials about adjuvant and neoadjuvant chemotherapy/EGFR-tyrosine kinase inhibitor (EGFR-TKI) are being conducted by CTONG (Figure 2). Adjuvant cisplatin-based chemotherapy is recommended for routine use in patients with Stage IIA, IIB, and IIIA NSCLCs after complete resection and thus cisplatin and vinorelbine combination act as standard therapy. In addition, activating somatic mutations of tyrosine kinase domains of EGFR have been characterized in a subset of patients with advanced NSCLC. The EGFR mutation rate was 40-50% in Asia NSCLC population. Patients harboring these mutations show excellent response to EGFR-TKIs. In Sep 2011, investigator-initiated multicenter randomized phase III clinical trial in China (Adjuvant, CTONG 1104) initiated adjuvant gefitinib studies in EGFR mutant NSCLC, when Japan (IMPACT; WJOG6401L) has a similar design at the same time (16). A total number of 220-230 completely resected IIIA-II/N2-N1 NSCLC patients with EGFR mutation would be randomized (1:1) into gefitinib versus combination of vinorelbine plus platinum group as adjuvant treatment. The primary endpoint is DFS. Despite of competition about patients enrolling between Japan and China, the final data will be independently published but pooled in collaboration. At the beginning stage of CTONG 1104, the enrollment was slow due to single origin of candidates. Following with researcher conferences, further coordination and communications between PIs, and promotion of multi-center, enrollment began to accelerate apparently from Sep 2012 and has finished enrollment now (Figures 3,4).
It is imperative to unite regulatory authorities, clinical trial sponsors, collaborative research groups, research nurses, study coordinators and data managers together to expedite study approval for clinical trials. There are several time internals act as bottlenecks which restrict progressions of trials to a large extent, such as regulatory approval, ethics approval, protocol amendments, time for patient enrollment, just name a few. When designing trial protocols, researchers have compared between different clinical trials, like ACOSOG-Z0030, LCSG 821, JCOG 0802, CALGB 140503, CALGB 39802, CHEST, NATCH, CSLC 0501, CTONG 1103 and CTONG 1104 on respects of collaborative research groups, financial support, collection of current evidence, trial design and planning, regulatory affairs, trial period activities, follow-up and data management. These trials have demonstrated surgeons’ ability to acquire fresh tissues when operating for central specimen bank storage. Surgeons have taken on the complex task of obtaining patients’ written informed consents for tissue acquisition and laboratory investigations. These trials enable patients to obtain easier access to innovative therapies and enhance the efficiency and quality of clinical researches, thus requiring surgeons taking more responsibility to successfully accrue patients with early-stage diseases. Effective cooperative group system would throw highlight on the promising future of thoracic malignancy therapy.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China [81001031 to W.Z. Zhong, 81372285 to W.Z. Zhong]; grant S2013010016354 from the Natural Science Foundation of Guangdong.
Disclosure: The authors declare no conflict of interest.
References
- Wakelee H, Kernstine K, Vokes E, et al. Cooperative group research efforts in lung cancer 2008: focus on advanced-stage non-small-cell lung cancer. Clin Lung Cancer 2008;9:346-351. [PubMed]
- Wakelee H, Langer C, Vokes E, et al. Cooperative group research efforts in lung cancer: focus on early-stage non-small-cell lung cancer. Clin Lung Cancer 2008;9:9-15. [PubMed]
- Wakelee H, Loo BW Jr, Kernstine KH, et al. Cooperative group research efforts in thoracic malignancies 2009: a review from the 10th Annual International Lung Cancer Congress. Clin Lung Cancer 2009;10:395-404. [PubMed]
- Posther KE, Wells SA Jr. The future of surgical research: the role of the American College of Surgeons Oncology Group. Eur J Surg Oncol 2005;31:695-701. [PubMed]
- Shimoyama M, Fukuda H, Saijo N, et al. Japan Clinical Oncology Group (JCOG). Jpn J Clin Oncol 1998;28:158-62. [PubMed]
- Subramanian J, Regenbogen T, Nagaraj G, et al. Review of ongoing clinical trials in non-small-cell lung cancer: a status report for 2012 from the ClinicalTrials.gov Web site. J Thorac Oncol 2013;8:860-5. [PubMed]
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [PubMed]
- Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 2006;355:983-91. [PubMed]
- Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [PubMed]
- Wente MN, Seiler CM, Uhl W, et al. Perspectives of evidence-based surgery. Dig Surg 2003;20:263-9. [PubMed]
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study J Clin Oncol 2007;25:4993-7. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [PubMed]
- Wu YL, Zhou Q. Clinical trials and biomarker research on lung cancer in China. Expert Opin Ther Targets 2012;16 Suppl 1:S45-50. [PubMed]
- Mitsudomi T, Suda K, Yatabe Y. Surgery for NSCLC in the era of personalized medicine. Nat Rev Clin Oncol 2013;10:235-44. [PubMed]