Safety and efficacy of aprepitant as mono and combination therapy for the prevention of emetogenic chemotherapy-induced nausea and vomiting: post-marketing surveillance in China
Introduction
Nausea and vomiting are common adverse events (AE) associated with cancer chemotherapy, affecting approximately 70% of treated patients, despite significant improvements in treatment options available for the management of cancer (1,2). Chemotherapy-induced nausea and vomiting (CINV) has been found to negatively impact the patients’ quality of life (QoL), leading to loss of appetite, general fatigue, constipation (3) and hospitalization (4). However, CINV is preventable (5,6) and neurokinin-1 (NK-1) receptor antagonists as combination antiemetic therapy with a serotonin receptor (5HT3) antagonist and dexamethasone can be used to prevent CINV associated with moderate and highly emetogenic chemotherapy (7).
Previous studies have shown that compared to moderate emetogenic chemotherapy (8,9), patients treated with high emetogenic therapy were at a greater risk of overall and delayed CINV (10,11), which may reach an incidence rate of >90% if suitable anti-emetic therapy is not prescribed (12). Given the role of NK-1 receptors (13) in the induction of delayed nausea and vomiting, the European Society of Medical Oncology (ESMO), the Multinational Association of Support Care Cancer (MASCC) and National Comprehensive Cancer Network (NCCN) guidelines recommend administering a triple combination consisting of a 5HT3 receptor antagonist, dexamethasone and a NK-1 receptor antagonist for patients receiving highly emetogenic chemotherapy (14,15).
Aprepitant is a NK-1 receptor antagonist commonly used for the prevention of CINV (16) and in combination with a 5HT3 receptor antagonist and dexamethasone has been shown to have potent anti-emetic activity in a pooled analysis of two phase III clinical trials (17). In these studies, aprepitant demonstrated significant efficacy in the prevention of acute or delayed nausea and vomiting associated with highly or moderate emetogenic chemotherapy therapy (17). In addition, in the aprepitant group, women had a greater overall complete response (CR) compared to men, which suggested the administration of aprepitant may be beneficial in preventing CINV in female patients receiving highly emetogenic chemotherapy (17). A phase III trial revealed that aprepitant combined with ondansetron, with or without dexamethasone, may be an effective treatment regimen for the prevention of CINV in pediatric patients receiving highly or moderate emetogenic chemotherapy (18).
It has also been reported, however, that aprepitant may influence the toxicity and the efficacy of concomitantly administered drugs, since a recent systematic review evaluated the possible pharmacokinetic drug interactions with aprepitant and fosaprepitant, and concluded that concurrent administration of aprepitant, ifosfamide, oxycodone, quetiapine, selective serotonin reuptake inhibitors/serotonin-norepinephrine reuptake inhibitors and warfarin may lead to AEs including neurotoxicity, a decreased respiratory rate, somnolence, vomiting and prothrombin time/international normalized ratio changes (19). A retrospective analysis of the NK-1 receptor antagonist, fosaprepitant, found that administration of fosaprepitant and anthracyclines through the same peripheral vein may cause a local reaction including swelling, extravasation and phlebitis at the infusion site (20). However, other AEs related to interactions of aprepitant with co-medications are rarely reported.
In the present study, we carried out a multicenter, single arm, prospective clinical study in China to evaluate the safety and efficacy of aprepitant in Chinese patients receiving highly emetogenic chemotherapy for the treatment of solid malignancies in the context of routine clinical practices. We also analyzed the factors associated with CR and assessed potential anti-emetic drug interactions associated with concomitant therapy. We present the following article in accordance with the TREND reporting checklist (available at http://dx.doi.org/10.21037/cco-20-160).
Methods
Study design
This multicenter, single arm, prospective, non-interventional surveillance study was conducted in 21 centers across China; with the objective of assessing the safety and efficacy of aprepitant in preventing CINV in patients with solid malignant tumors treated with highly emetogenic chemotherapy.
Inclusion criteria were: adult males or females ≥18 years of age; the patient must be willing to provide written informed consent; the patient was scheduled to receive his/her highly emetogenic chemotherapy; patient was treated with aprepitant for the first time; the patient was able to read, understand and complete the subject diaries; and the patient must be able to understand written Chinese and complete the subject diaries. No translations of the subject diaries other than those provided by the SPONSOR were permitted.
Exclusion criteria were: patients having any medical condition or concurrent use of medications, which may be a contraindication to the approved local prescribing information as per the investigator’s opinion; contraindication to aprepitant; patient had received a non-approved (investigational) drug within the last 4 weeks; and any condition which in the opinion of the investigator may confound the results of the survey or pose unwarranted risk in administering the study drug to the patient. In addition, patients were excluded if they had one or more of the following conditions: concurrent usage of pimozide, terfenadine, astemizole or cisapride and aprepitant was contraindicated due to hypersensitivity to any component of the product.
The enrollment period was 21 months and 10 days. Aprepitant was given with a 5HT3 antagonist plus a corticosteroid, but physicians prescribed aprepitant according to their discretion and in many cases a non-recommended dose/regimen of aprepitant was employed.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the independent ethics committees of all the participating centers and informed consent was taken from all the patients. The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) registration number is EUPAS29952.
Study population
The study included male and female patients aged ≥18 years. All enrolled patients provided written informed consent to participate, were scheduled to receive highly emetogenic chemotherapy regimens, were aprepitant naïve and able to read and understand Chinese to complete patient diaries. Patients could, with mutual agreement, withdraw from the study for reasons deemed justified by the investigator.
Treatment and surveillance
All included patients received CINV prophylaxis, which is defined as antiemetic therapy prescribed to prevent the occurrence of nausea or vomiting administered according to the investigator’s orders. Based on NCCN/ASCO guidelines, the CINV prophylaxis medication consisted of a 5HT3 antagonist administered before chemotherapy on Day 1 and a corticosteroid administrated on Days 1 to 3 or on Day 4. The recommended dose of aprepitant was 125 mg orally 1 h prior to chemotherapy treatment (Day 1) and 80 mg orally once daily in the morning on Days 2 and 3.
For prior chemotherapy treatments including initiation time, drug, dosage/dose unit and the route of administration, the investigator investigated medical records and included prior medications taken by the patient within 7 days before the administration of aprepitant.
For concomitant medication, all were taken by patients from the time of the first dose of aprepitant to 5 days after the last dose; data were extracted from the patient’s medical record or patient diaries together with dosing level. The concomitant medications were from two sources: (I) all concomitant medications taken in the hospital recorded in the patient medical record prior to discharge; (II) entries in the Medication Questionnaire of the patients’ dairies, which was reviewed by investigators when it was returned to the site prior to data entry into the CRF. The information included the chemotherapy administered, drug name, dosage and formulation of CINV prophylaxis or rescue medications administered, as well as other medications. Alcohol consumption was defined as drinking alcoholic beverages >10 times per week and history of alcohol consumption was evaluated as yes/no, >10 times per week and <10 times per week according to the patients information.
Rescue therapies were planned to be classified as: antihistamines (e.g., cetirizine, clementine, cyclizine, dexchlorpheniramine or meclizine), 5HT3 antagonists (e.g., granisetron, dolasetron, tropisetron, ramosetron or ondansetron), phenothiazines (e.g., metopimazine, prochlorperazine, fluphenazine, perphenazine, thiethylperazine, levomepromazine, or chlorpromazine), butyrophenones and butyrophenone derivatives (e.g., haloperidol or droperidol), benzamides (e.g., metoclopramide, levosulpiride, or alizapride), benzodiazepines (e.g., alprazolam, clonazepam, diazepam, lorazepam, midazolam), corticosteroids (e.g., prednisone, prednisolone, methylprednisolone, dexamethasone, or betamethasone), domperidone-antacids/proton pump inhibitors/histamine H2-receptor antagonists (e.g., bismuth subsalicylate, ranitidine, famotidine, chewable calcium carbonate, esomeprazole, omeprazole, pantoprazole), others including Traditional Chinese Medicine (e.g., olanzapine or hyoscine).
However, in order to reflect the real clinical situation, all patients who took at least one dose of aprepitant prior to receiving highly emetogenic chemotherapy were included and patients who provided consent received diaries including: (I) MASCC antiemesis Tool (MAT), (II) functional living index-emesis (FLIE) and (III) medication questionnaires after taking each dose of aprepitant. Patients were instructed and reminded to complete the diaries in a timely fashion. In addition, patients were contacted by investigators or qualified designees to assess AEs up to 14 days after the last dose of aprepitant. The investigators reviewed the medication questionnaires before they subsequently completed the documentations and then two researchers verified the data. An AE was defined as any unfavorable and unintended temporally change in the structure, function, or chemistry of the body without necessarily having a causal association with the use of aprepitant. A drug related AE was defined as an AE resulting from the use of aprepitant. The Medical Dictionary for Regulatory Activities (MedDRA), version 17.0, was used for AE coding. AEs were classified according to the MedDRA system organ class (SOC) and preferred term (PT).
Study endpoints
Primary endpoints were AEs, drug related AEs and serious AEs
To characterize the safety profile of aprepitant, the occurrence of any AEs, including drug related AEs and serious AEs (SAEs), and discontinuation of treatment due to AEs were monitored, recorded, and analyzed as the primary endpoints of the study.
Patients, who received at least one dose of aprepitant (EMEND®) were monitored for AEs (physical examination, vital signs, laboratory tests) throughout aprepitant treatment up to 14 days after the last dose of aprepitant was administered. Events related to the efficacy endpoint (vomiting, retching, nausea) were not defined as AEs during Day 1 until the morning of Day 6 (a total of 120 h), unless they met the definition of SAEs. All AEs were collected in person or via telephone, recorded in the patient’s medical record and reported by the investigators or the qualified designees. The investigators assessed the relationship to aprepitant. The main safety endpoints were: the patient proportion of one or more (I) AEs; (II) drug related AEs; (III) SAEs; (IV) discontinuation due to an AE.
Secondary endpoint: evaluation of the efficacy of aprepitant
Secondary endpoints were the evaluation of the efficacy of aprepitant during the acute phase (0–24 h), the delayed phase (25–120 h) and the total phase (0–120 h), and included CR, the proportion of patients with no vomiting and no clinically significant nausea, as well as no nausea and no impact on QoL (0–120 h).
In this study, the criteria to evaluate CINV was based on the MAT.
CR was defined as no vomiting, with no rescue therapy (rescue therapy was any medication administered to treat established nausea or vomiting).
No vomiting was defined as no vomiting, retching or dry heaves (including patients who received rescue therapy).
No nausea was defined as a MAT score of 0.
No clinically significant nausea was defined as a MAT score of 1–2.
No impact on QoL was defined as a FLIE score >6 in 7 subscales (>108 total points).
Vomiting assessment
A vomiting episode was defined as one or more continuous vomiting (expulsion of stomach contents through the mouth) or retches (an attempt to vomit that did not produce stomach contents; also referred to as dry heaves). The presence and number of vomiting episodes were recorded by patients in MAT. Definitions were also provided in MAT. The patients were educated to adhere to the protocol definition of vomiting episodes and to review these definitions as necessary.
Nausea assessment
Nausea was self-assessed using a 0–10 scale (MAT item 4 and 8) in the patients’ diaries. For example, “If you had nausea, please circle or enter the number that most closely resembles your nausea”.
Statistical analysis
Patient data are presented as frequencies and percentages. Safety and efficacy endpoints are given as descriptive statistics in numbers, observed rates and 95% confidence intervals (CIs). The CR rate of all included aprepitant medication regimens are presented as percentages with P values. After univariate logistic regression analysis of factors, a multivariate logistic regression analysis, with adjustments for the potential clinical factors including gender, age, cisplatin dosage and duration of usage, antiemetic-therapy regimens and alcohol consumption was conducted to identify factors associated with CRs in 0–120 h, 0–24 h and 25–120 h treatment intervals, with odds ratios (ORs) and 95% CIs determined. All statistical tests were performed using the “R” software statistical package and P values <0.05 were considered to be statistically significant.
Result
Demographic and baseline characteristics of patients
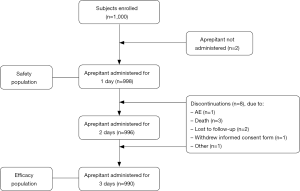
In the 21 participating centers, a total of 1,000 Chinese patients with solid malignant tumors were enrolled [median 54.0 years (range, 18–85); 482 (48.2%) females; BMI: 23.03±3.22 kg/m2]. Of the 1,000 patients, 998 (99.8%) received at least 1 dose of aprepitant and were included in the safety analysis; 990 (99.0%) received at least 3 doses of aprepitant and were included in the safety analysis (Figure 1). From all included patients 982 (98.2%) completed the MASCC anti-emesis tool, 980 (98.0%) the medication questionnaire and the FLIE questionnaire were completed by 972 (97.2%) patients. Of the patients, 14.5% had a history of alcohol consumption and 12.2% a history of CINV (Table 1).

Full table
Most of the patients were lung cancer (50.5%) and breast cancer (23.8%) cases and the primary cancer diagnosis phase for all patients was mainly stage III–IV (60.7%).
Of the patients, 76.2% had received ≥1 prior medication (except chemotherapy including herbal and traditional medicine (38.6%), corticosteroids for systemic use (24.9%), and drugs for acid related disorders (23.8%). A total of 998 patients received aprepitant on Day 1 before chemotherapy was initiated; 996 patients and 991 patients continued to receive therapy at 80 mg QD on Day 2 and Day 3. In addition, most patients received cisplatin bases chemotherapies [723 (72.3%)]. Other combination therapies administered to patients accounted for <10% of all treatments (Table 1).
Safety of aprepitant
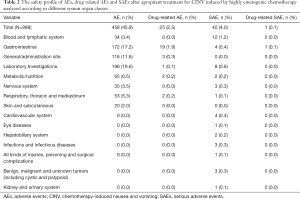
Overall, 458 (45.9%) patients in the safety population (n=998) reported ≥1 AE. The rate of occurrence of ≥1 drug related AEs or SAEs was 2.5% and 4.0%, respectively. Only one patient with a SAE (0.1%) did not complete the study. AEs listed according to different system organ classes are shown in Table 2.
Full table
Within alterations detected by laboratory parameters of urinalysis, hematology, and blood chemistry (19.6%; 95% CI: 17.2–22.2%) decreases in white blood cell (WBC) (11.2%) and neutrophil (5.9%) counts were the most common AEs. The second highest AE incidence was seen in the gastrointestinal system organ class, with 17.2% (95% CI: 14.9–19.7%) of patients experiencing a gastrointestinal AE. These included constipation (6.7%), nausea (4.8%), vomiting (2.2%) or diarrhea (1.9%). Most drug-related AEs were associated with the gastrointestinal system organ class (1.9%; 95% CI: 1.2–3.0%) (Table 2).
Of the cohort of 40 patients who experienced SAEs, blood related disorders were the major SAEs (1.2%; 95% CI: 0.6–2.1%) including bone marrow failure (1.1%) and febrile neutropenia (0.2%) (Table 2). There was only one SAE of gastrointestinal disorder was judged related to study drug and not an ECI at the discretion of investigator, but this subject was recovering from this event. All of the other SAEs were not related to the study drug. There were 4/998 (0.4%) deaths reported in the study. All 4 deaths were unrelated to the study drug.
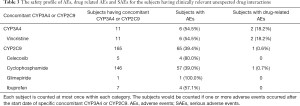
Finally, the clinically relevant unexpected drug interactions between aprepitant and substrates of CYP3A4 (vinblastine and vincristine) and CYP2C9 (i.e., warfarin, tolbutamide and oral hypoglycemics) were assessed in this study.
Table 3 presents the summary of clinically relevant unexpected drug interactions between aprepitant and substrates of CYP3A4 and CYP2C9. There was no subject treated with vinblastine concomitantly in the study. In 11 subjects treated with vincristine concomitantly, 6 subjects reported one or more AEs and 2 subjects reported one or more drug-related AEs. In 165 subjects treated with CYP2C9 substrates concomitantly, 65 (39.4%) subjects reported one or more AEs and only 1 subject reported one or more drug-related AEs, which are consistent with the safety profile of the previous studies. Therefore, there were no clinically relevant unexpected drug interactions noted in this study.
Full table
Aprepitant efficacy evaluation
After aprepitant therapy, we analyzed the complete response (CR) of 990 patients 0–120 h from the initiation of highly emetogenic chemotherapy. The overall rate of CR was 41.0% (95% CI: 37.9–44.1%) in the population of patients who received different drug combinations (n=990, efficacy population). The CR rates in the acute (0 to 24 h) and delayed (25 to 120 h) phases are shown in Table 4. The CR rate in the acute phase was significantly greater compared to the delayed phase (66.0% vs. 46.5%, P<0.001).

Full table
The proportion of patients with no vomiting from 0 to 24 h (acute) and from 25 to 120 h (delayed) as well as the overall study period (0–120 h), no nausea (0–120 h)
The overall CR rates and no vomiting or no nausea in the acute (0 to 24 h) and delayed (25 to 120 h) phases as well as during the overall study period (0–120 h) in patients treated with highly emetogenic chemotherapies and anti-emetic drugs as solely aprepitant, dual, triple, quadruple and quintuplet therapies were 66.0%, 46.5% and 41.0%, 66.1%, 46.5%, 41.0%, 100.0%, 48.3% and 41.1%, whereas for no impact on QoL they were 92.0%, 48.0% and 64.4%, respectively (Table 4).
Rates of no vomiting and no nausea after aprepitant and/or combined with other anti-emetic drugs
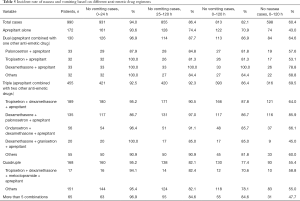
Further analysis showed that 172 (17.4%) of patients received aprepitant as monotherapy, 130 (13.1%) received dual anti-emetic drugs including aprepitant, 455 (46.0%) triple and 168 (17.0%) quadruple, while quintuplet anti-emetic drugs were only administered to 65 (6.6%) highly emetogenic chemotherapy treated patients (Table 5).
Full table
From Table 5, it is clear that the rate of no vomiting in patients receiving triple anti-emetic drugs in the acute and delayed phase was 92.5% and 92.3%, respectively. The overall no vomiting and no nausea rate (0–120 h) in patients receiving the triple anti-emetic drug schemes (aprepitant combined with 2 other anti-emetic drugs) were 86.4% and 69.5%, which were higher than the responses to aprepitant monotherapy of 70.9% and 43.0% (both P<0.001), dual anti-emetic drug schemes (aprepitant combined with one other anti-emetic drug) (86.9% and 64.6%, P=0.872, P=0.296), and quadruple anti-emetic drug schemes (aprepitant combined with 3 other anti-emetic drugs) (77.4% and 55.4%, P=0.007, P=0.001). The above findings clearly indicated that triple anti-emetic combination drugs had superior efficacy in reducing nausea and vomiting in the 1–120 h period compared to other regimens.
In addition, dexamethasone combined with aprepitant and palonosetron produced a higher rate of no nausea and no vomiting during the 0–120 h period (86.7% and 85.9%).
In comparison with palonosetron combined with aprepitant, tropisetron combined with aprepitant, or dexamethasone combined with tropisetron, we found that the dexamethasone and aprepitant combination increased the rate of no vomiting to 100.0% and no nausea rate to 78.8%, respectively, indicating that dexamethasone was the most suitable co-medication with aprepitant (Table 5).
Analysis of the CR rates of aprepitant protection against vomiting and nausea induced by combination chemotherapy
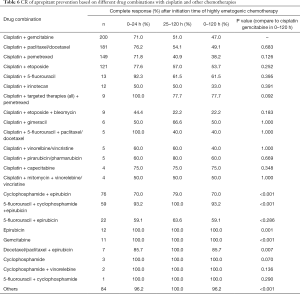
From analysis of the CR rates of aprepitant protection effect against vomiting and nausea induced by cisplatin combination chemotherapy, we found that the CR rates, not only in the acute phase, but also in the delayed phase and in the overall study period (0–120 h) were different and the average CR rates were lower than for other chemotherapies, based on various combination treatment regimens with cisplatin. However, when compared to the most common chemotherapy (cisplatin + gemcitabine), the rates of CR after aprepitant prevention treatment were not significantly different for each type of cisplatin combination therapy (Table 6).
Full table
In general, aprepitant prevention treatments for cisplatin and cisplatin combination chemotherapies produced lower CR rates than prevention treatments for other chemotherapies, which reached mostly 100% (Table 6).
Factors affecting CR determined by multivariable regression analysis
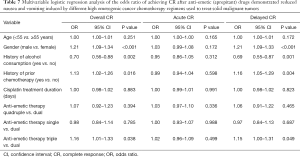
Using multivariable regression analysis, we found that triple anti-emetic therapy (OR 1.16; P=0.038), male gender (OR 1.21; P<0.001), history of alcohol consumption (yes vs. no) (OR 0.70; P=0.002) and a history of prior chemotherapy regarding any kind of agent (OR 1.13; P=0.016) were significant CR influencing factors in the overall phase (0–120 h); triple anti-emetic therapy (OR 1.15; P=0.049), male patients (OR 1.21; P<0.001), history of alcohol consumption (yes vs. no) (OR 0.69; P=0.001) and a history of chemotherapy (OR 1.16; P=0.004) were shown to be significantly associated with CR during the delayed phase. In contrast, none of the analyzed factors affected CR significantly during the acute phase. For the CR influencing factor analysis in different phases dual anti-emetic therapy, female, no alcohol consumption, and no history of the prior chemotherapy were referenced (Table 7).
Full table
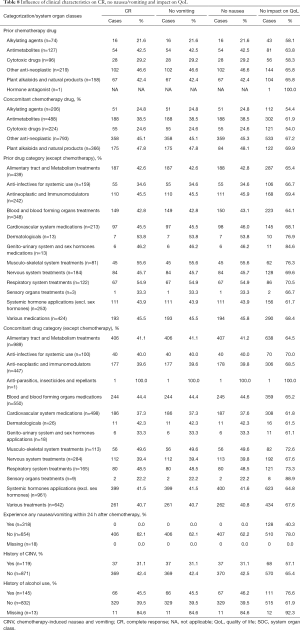
A history of prior chemotherapy (OR 1.13; P=0.016) was a significant risk factor for CR in the present study. We further analyzed the effect of prior chemotherapy on CR. Among prior chemotherapies, the use of anti-neoplastic drugs other than alkylating agents, antimetabolites, cytotoxic drugs, plant alkaloids and natural products produced a proportion of patients with CR of 46.6%, no vomiting, no nausea (46.6%, for all) and no impact on QoL (65.8%), whereas the lowest CR, no vomiting and nausea rates were found with alkylating agents (21.6%, 21.6% and 21.6%, respectively) (Table 8). These results indicated that prior administration of chemotherapy drugs affected the CR of patients treated with aprepitant after highly emetogenic chemotherapy therapy. Patients who received concomitant drugs also showed a difference in CR, vomiting and nausea rates based on different medications. This finding was especially true after the use of prior medications related to dermatological, musculo-skeletal and respiratory system treatments in which the CRs were in contrast to other concomitant medications >50% (Table 8).
Full table
Discussion
Aprepitant is part of an antiemetic regimen consisting of a 5HT3 antagonist/dexamethasone regimen with an overall CR (0–120 h) of 41.0% in our study, which was comparatively lower than in other reported comparable studies 48.9% (21) and 62.8% (22). The rate of no vomiting in our study was also lower (41.0% from 0 to 120 h) compared with Western 81.5% (21) 76.2% (22) and other Chinese studies with rates of 64% (23) and 74% (24) reported.
The difference between this study and other published data is that our study evaluated “real world” use of aprepitant, and the dosing/regimen were at the discretion of prescribing physicians. Since many of them prescribed aprepitant monotherapy, the efficacy was lower, because patients did not receive the recommended regimen. Another possible reason may be that patients enrolled in our study had already received prior chemotherapy and other organ system related treatments, which seriously affected CR and the no vomiting and no nausea rates (Table 8). Alkylating agents and cytotoxic drugs could have predisposed these patients to vomiting, with gender and age playing roles in driving CR (patients aged <55 years and females had a lower CR). Similar results were reported in a Japanese study, in which aprepitant therapy was more effective in preventing CINV in male patients, who achieved higher no vomiting and CR rates than females (25). Also other studies noted that in addition to age and a history of nausea/vomiting, especially women, younger patients and those who don’t drink much alcohol, had an elevated risk of experiencing CINV (26-28), which is in agreement with the findings of the present study.
Percentages of patients with AEs (45.9%), drug related AEs (2.5%), SAEs (4.0%) and drug related SAEs (0.1%) were mainly lower than in previous studies with 62.8% AEs, 7.2% drug related AEs, 2.8% SAEs (22), 40% AEs, 11.7% drug related AEs and 1.3% SAEs (24).
In the present study, prior or concomitant therapy with anti-metabolite plant alkaloids impacted on the QoL of many patients, but rescue medication was not required in the majority of cases (98.3% and 94.7%), findings similar to other published data (29,30). Aprepitant based triple therapy did not impact on the QoL of patients in contrast to solely ondansetron and dexamethasone (31) administrations, which is in agreement with the guidelines which recommend the addition of an NK-1 receptor antagonist to a 5HT3 antagonist + corticosteroid combination to improve QoL.
A combination of chemotherapeutic agents may have an impact on CR, which is dependent on their emetogenic potential (32), which was reflected in the high CR rates for chemotherapy regimens other than cisplatin. In the present study, highly emetogenic chemotherapies and moderate emetogenic cisplatin containing chemotherapies led to low CRs, with the lowest CR rate observed in patients treated with cisplatin + 5-fluorouracil + epirubicin/doxorubicin. After low dose cisplatin, highly emetogenic chemotherapy has been demonstrated to induce nausea and vomiting in both patients and animal models, an action which increases significantly with increasing dosage (33). This treatment causes acute and delayed vomiting (33,34), but the time of onset of nausea is shorter compared with vomiting (33). In our study, this trend between medication duration and the dose of cisplatin (30–110 mg) was not seen (data not shown). The CRs achieved with different durations of cisplatin treatment were similar, suggesting that aprepitant was equally effective regardless of the duration of cisplatin treatment.
We found, that dexamethasone was the most suitable co-medication for aprepitant, which is in agreement with the NCCN recommendation of combination therapy over monotherapy for CINV management (35,36), a finding underlined by the fact that dexamethasone is an integral component of almost all antiemetic drug regimens (32).
Taken together aprepitant was found to be safe and tolerable in Asian (35,37,38) and Western populations (22,36), findings confirmed in the present study involving Chinese patients.
However, there were a number of limitations to our study. The results are confounded by the variability index usage and the lack of a control group. First, self-reporting of data using MASCC and FLIE questionnaires may possibly have led to under/over-reporting of the efficacy or safety variables. In addition, this was not a randomized study. Second, an element of subjective bias cannot be ruled out during the documentation of symptoms; therefore, the results must be interpreted with a degree of caution. However, the objective of the study was to look at aprepitant usage in the context of routine clinical practice in which there is inherent variability in the concomitant use of corticosteroids and other drugs.
Conclusions
In conclusions, the present study showed that aprepitant for the prevention of CINV associated with highly emetogenic chemotherapy was generally safe and well tolerated with only 1 drug related SAE, which was resolved during treatment. The combination of anti-emetic drugs with aprepitant affected positively the CR rate in highly emetogenic chemotherapy treated patients. The results also suggest that aprepitant use in combination with standard anti-emetic drugs should be selected on the basis of the emetogenic potential of the administered chemotherapy.
Acknowledgments
We thank Amit Bhat, PhD and Priyanka Bannikoppa, PhD (Indegene Pvt Ltd, Bangalore, India) for providing medical writing support and technical assistance in the preparation of this manuscript, thanks to funding from MSD China.
Funding: This study was funded by MSD China.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at http://dx.doi.org/10.21037/cco-20-160
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cco-20-160
Peer Review File: Available at http://dx.doi.org/10.21037/cco-20-160
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco-20-160). All authors report other from MSD China, during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the independent ethics committees of all the participating centers and informed consent was taken from all the patients. The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) registration number is EUPAS29952.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mardas M, Madry R, Stelmach-Mardas M. Link between diet and chemotherapy related gastrointestinal side effects. Contemp Oncol (Pozn) 2017;21:162-7. [Crossref] [PubMed]
- Ryan JL. Treatment of Chemotherapy-Induced Nausea in Cancer Patients. Eur Oncol 2010;6:14-6. [PubMed]
- Ishikawa A, Ohara G, Nakazawa K, et al. Chemotherapy-induced complications in patients with lung cancer: An evaluation by pharmacists. Mol Clin Oncol 2013;1:65-8. [Crossref] [PubMed]
- Schwartzberg L, Harrow B, Lal LS, et al. Resource Utilization for Chemotherapy-Induced Nausea and Vomiting Events in Patients with Solid Tumors Treated with Antiemetic Regimens. Am Health Drug Benefits 2015;8:273-82. [PubMed]
- Hurvitz S, Guerin A, Brammer M, et al. Investigation of adverse-event-related costs for patients with metastatic breast cancer in a real-world setting. Oncologist 2014;19:901-8. [Crossref] [PubMed]
- Lau PM, Stewart K, Dooley M. The ten most common adverse drug reactions (ADRs) in oncology patients: do they matter to you? Support Care Cancer 2004;12:626-33. [PubMed]
- Zhang Y, Yang Y, Zhang Z, et al. Neurokinin-1 Receptor Antagonist-Based Triple Regimens in Preventing Chemotherapy-Induced Nausea and Vomiting: A Network Meta-Analysis. J Natl Cancer Inst 2016;109:djw217. [Crossref] [PubMed]
- Tsuji Y, Baba H, Takeda K, et al. Chemotherapy-induced nausea and vomiting (CINV) in 190 colorectal cancer patients: a prospective registration study by the CINV study group of Japan. Expert Opin Pharmacother 2017;18:753-8. [Crossref] [PubMed]
- Escobar Y, Cajaraville G, Virizuela JA, et al. Incidence of chemotherapy-induced nausea and vomiting with moderately emetogenic chemotherapy: ADVICE (Actual Data of Vomiting Incidence by Chemotherapy Evaluation) study. Support Care Cancer 2015;23:2833-40. [Crossref] [PubMed]
- Nitta H, Baba H, Sugimori K, et al. Chemotherapy-induced Nausea and Vomiting in Patients with Hepatobiliary and Pancreatic Cancer Treated with Chemotherapy: A Prospective Observational Study by the CINV Study Group of Japan. Anticancer Res 2016;36:1929-35. [PubMed]
- Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3 (Alliance). Support Care Cancer 2016;24:2661-7. [Crossref] [PubMed]
- Rapoport BL. Delayed Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Incidence, and Current Management. Front Pharmacol 2017;8:19. [Crossref] [PubMed]
- Navari RM. 5HT3 receptors as important mediators of nausea and vomiting due to chemotherapy. Biochim Biophys Acta 2015;1848:2738-46. [Crossref] [PubMed]
- Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119-33. [Crossref] [PubMed]
- Berger MJ, Ettinger DS, Aston J, et al. NCCN Guidelines Insights: Antiemesis, Version 2.2017. J Natl Compr Canc Netw 2017;15:883-93. [Crossref] [PubMed]
- Shadle CR, Lee Y, Majumdar AK, et al. Evaluation of Potential Inductive Effects of Aprepitant on Cytochrome P450 3A4 and 2C9 Activity. J Clin Pharmacol 2004;44:215-23. [Crossref] [PubMed]
- Hesketh PJ, Grunberg SM, Herrstedt J, et al. Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support Care Cancer 2006;14:354. [Crossref] [PubMed]
- Kang HJ, Loftus S, Taylor A, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting in children: a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:385-94. [Crossref] [PubMed]
- Patel P, Leeder JS, Piquette-Miller M, et al. Aprepitant and fosaprepitant drug interactions: a systematic review. Br J Clin Pharmacol 2017;83:2148-62. [Crossref] [PubMed]
- Hegerova LT, Leal AD, Grendahl DC, et al. An analysis of fosaprepitant-induced venous toxicity in patients receiving highly emetogenic chemotherapy. Support Care Cancer 2015;23:55-9. [Crossref] [PubMed]
- Meattini I, Francolini G, Scotti V, et al. Aprepitant as prophylaxis of chemotherapy-induced nausea and vomiting in anthracyclines and cyclophosphamide-based regimen for adjuvant breast cancer. Med Oncol 2015;32:80. [Crossref] [PubMed]
- Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer 2010;18:423-31. [Crossref] [PubMed]
- Hu W, Fang J, Nie J, et al. Addition of aprepitant improves protection against cisplatin-induced emesis when a conventional anti-emetic regimen fails. Cancer Chemother Pharmacol 2014;73:1129-36. [Crossref] [PubMed]
- Hu Z, Cheng Y, Zhang H, et al. Aprepitant triple therapy for the prevention of chemotherapy-induced nausea and vomiting following high-dose cisplatin in Chinese patients: a randomized, double-blind, placebo-controlled phase III trial. Support Care Cancer 2014;22:979-87. [Crossref] [PubMed]
- Takemoto H, Nishimura J, Komori T, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy in the SENRI trial: analysis of risk factors for vomiting and nausea. Int J Clin Oncol 2017;22:88-95. [Crossref] [PubMed]
- Boccia R. Chemotherapy-Induced Nausea and Vomiting: Identifying and Addressing Unmet Needs. J Clin Outcomes Manage 2013;20:377-84.
- Molassiotis A, Aapro M, Dicato M, et al. Evaluation of Risk Factors Predicting Chemotherapy-Related Nausea and Vomiting: Results From a European Prospective Observational Study. J Pain Symptom Manage 2014;47:839-48.e4. [Crossref] [PubMed]
- Sekine I, Segawa Y, Kubota K, et al. Risk factors of chemotherapy-induced nausea and vomiting: Index for personalized antiemetic prophylaxis. Cancer Sci 2013;104:711-7. [Crossref] [PubMed]
- Zhang L, Lu S, Feng J, et al. PD Quality of life (QOL) evaluation of patients in a phase 3 study comparing NEPA with an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV). Ann Oncol 1548;2017. [Crossref]
- Yeo W, Mo FKF, Suen JJS, et al. A randomized study of aprepitant, ondansetron and dexamethasone for chemotherapy-induced nausea and vomiting in Chinese breast cancer patients receiving moderately emetogenic chemotherapy. Breast Cancer Res Treat 2009;113:529-35. [Crossref] [PubMed]
- Martin AR, Carides AD, Pearson JD, et al. Functional relevance of antiemetic control: experience using the FLIE questionnaire in a randomised study of the NK-1 antagonist aprepitant. Eur J Cancer 2003;39:1395-401. [Crossref] [PubMed]
- Jordan K, Sippel C, Schmoll HJ. Guidelines for Antiemetic Treatment of Chemotherapy-Induced Nausea and Vomiting: Past, Present, and Future Recommendations. Oncologist 2007;12:1143-50. [Crossref] [PubMed]
- Kenward H, Pelligand L, Elliott J. Assessment of low-dose cisplatin as a model of nausea and emesis in beagle dogs, potential for repeated administration. Exp Brain Res 2014;232:2685-97. [Crossref] [PubMed]
- Liaw CC, Wang CH, Chang HK, et al. Prevention of acute and delayed cisplatin-induced nausea and vomiting with intravenous ondansetron plus intravenous dexamethasone. Chang Gung Med J 2000;23:413-9. [PubMed]
- Kitamura H, Takahashi A, Hotta H, et al. Palonosetron with aprepitant plus dexamethasone to prevent chemotherapy-induced nausea and vomiting during gemcitabine/cisplatin in urothelial cancer patients. Int J Urol 2015;22:911-4. [Crossref] [PubMed]
- Micha JP, Rettenmaier MA, Brown JV 3rd, et al. A Randomized Controlled Pilot Study Comparing the Impact of Aprepitant and Fosaprepitant on Chemotherapy Induced Nausea and Vomiting in Patients Treated for Gynecologic Cancer. Int J Gynecol Cancer 2016;26:389-93. [Crossref] [PubMed]
- Hingmire S, Raut N. Open-label observational study to assess the efficacy and safety of aprepitant for chemotherapy-induced nausea and vomiting prophylaxis in Indian patients receiving chemotherapy with highly emetogenic chemotherapy/moderately emetogenic chemotherapy regimens. South Asian J Cancer 2015;4:7-10. [Crossref] [PubMed]
- Kim JE, Jang JS, Kim JW, et al. Efficacy and safety of aprepitant for the prevention of chemotherapy-induced nausea and vomiting during the first cycle of moderately emetogenic chemotherapy in Korean patients with a broad range of tumor types. Support Care Cancer 2017;25:801-9. [Crossref] [PubMed]