Systemic therapies for melanoma brain metastases: which drug for whom and when?
Introduction
Metastatic melanoma (stage IIIc unresectable or stage IV) is associated with a poor prognosis, and until recently, a median overall survival (OS) of 6-9 months (1). For patients who develop brain metastasis, the median survival is reduced to 17 to 22 weeks (2,3). The incidence of overt brain metastasis at first presentation is approximately 20% and approximately 50% of stage IV melanoma patients develop brain metastases during the course of their disease (3). The presence of BRAF or NRAS mutations increases the risk of developing brain metastasis at first diagnosis of metastatic disease (4). In addition to reduced life expectancy, patients with symptomatic lesions experience neurocognitive decline and poor quality of life (5). The aim of treatment is to reduce neurological symptoms, minimise cognitive decline and improve survival. Recently, incorporation of such patients into clinical trials assessing newer systemic therapies has provided increased therapeutic options, and raises questions regarding the optimal selection and combinations of treatment modalities for brain metastasis.
Prognostic and predictive factors for brain metastasis in melanoma
Clinicopathological factors predictive of short central nervous system (CNS) metastasis-free interval were M1b/M1c disease, head and neck primaries, superficial spreading/nodular subtypes and elevated baseline serum lactate dehydrogenase (LDH) (6,7). Molecular predictors of brain metastases are emerging and include the association of BRAF, NRAS and PTEN mutations and survival. The presence of a BRAF mutation was predictive of increased response rate to BRAF targeted therapy and was associated with significantly improved survival in comparison with BRAF wild-type patients and hence also of prognostic significance (8). There have been inconsistent reports of associations of NRAS mutations with prognosis in melanoma (9). Several studies have reported the NRAS mutations to be significantly associated with poorer prognostic features in the primary (10), shorter melanoma-specific survival from primary diagnosis (10) and poorer survival from stage IV diagnosis (4). The PTEN mutations (loss of expression of tumour suppressor PTEN) are mutually exclusive with NRAS mutations, are associated with BRAF V600 mutations and BRAF/NRAS wild type tumours and are predictive of shorter OS and shorter time to brain metastases (11).
In large retrospective studies of melanoma patients with brain metastases, poor prognostic factors associated with worse survival were >3 parenchymal brain lesions, leptomeningeal disease, brain lesions developing concurrently with extracranial disease or while on systemic therapy for extracranial disease, poor performance status (Karnofsky performance status <70%), elevated pre-treatment LDH levels and Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) class III (12-15). Patients with limited intracranial disease treated with neurosurgery or stereotactic radiosurgery (SRS) had better survival in comparison with those with diffuse disease and who received whole brain radiotherapy (WBRT) (16).
Systemic therapies
Until recently, systemic therapy in melanoma brain metastasis was limited to using chemotherapeutic agents after failure of local therapies. This is because melanoma cells are resistant to chemotherapy and response rates in the brain have been poor (17-20). It has been proposed that the lack of activity is due to inadequate penetrance of blood brain barrier and expression of active efflux transporters such as P-glycoprotein and breast cancer resistance protein (BCRP) (21). However, the extracranial activity is also limited with these drugs. The BRAF inhibitors dabrafenib, vemurafenib and the anti-CTLA4 antibody ipilimumab have shown activity in such patients with active (untreated or progressed after previous therapy) melanoma brain metastases in phase 2 studies, with increased response rates and improved survival compared with historic controls (17,22,23). The selection of an appropriate systemic agent depends on the patient’s performance status, presence of medical co-morbidities precluding use of a drug, number and size of brain metastases, neurological symptoms and complications, burden of extracranial metastases and tumour mutation status (24,25).
Somatic mutations in melanoma and clinicopathological associations
Melanoma is associated with a high burden of somatic mutations and aberrations (26), the most frequent and well-known mutations are the BRAF and NRAS mutations, occurring in 35-45% and 15-25% of melanoma patients (8,10,27,28), along with the KIT mutation, which occur in 3-4% of western populations with melanoma (27). The BRAF and NRAS mutations are the focus of targeted drug therapy in melanoma.
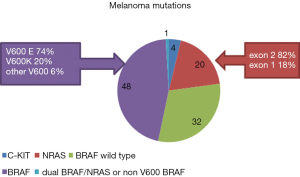
The serine-threonine kinase BRAF is a component of the RAF-MEK-MAPK pathway [known as the mitogen activated protein kinase (MAPK) pathway] and point mutations in the BRAF gene occur in 33-47% of primary melanoma and 41-55% of metastatic melanomas (8,27,29) (Figure 1). The most common BRAF mutation is V600E, occurring in 74-76% of all BRAF mutations (8,27), and is due to the substitution of the amino acid valine with glutamic acid at position 600 of the BRAF protein, resulting in activation of downstream kinases. The BRAF V600K [occurring in 10-20% of BRAF mutations (8,27)] results from substitution of valine with lysine at position 600 of the BRAF protein. Other V600 mutations are rare (6%) (4,8,9). DNA sequencing (using Sanger sequencing, often combined with high resolution melt analysis to increase sensitivity) or pyro-sequencing, immunohistochemistry, as well as PCR based testing platforms are used to detect these mutations in tumour samples with varying sensitivities and specificities (30). The Roche Cobas 4800 is a real-time PCR technique which detects BRAF mutations V600E, V600K, V600D, with only high sensitivity and specificity for the BRAF V600E mutations (31). Immunohistochemistry utilises a specific monoclonal antibody (VE1) for detecting BRAF V600E protein in melanoma and is a highly sensitive (97%), specific (98%), rapid and a cost-effective analysis, utilising minimal tissue (32,33). It provides a result at the time of pathological diagnosis and accurately discriminates BRAF V600E mutations from non-V600E mutations. There are many methods of BRAF mutation testing that are approved by country-specific laboratory agencies [e.g., Clinical Laboratory Improvement Amendments (CLIA), USA], and the role for other bodies e.g., the Food and Drug Administration (FDA) is less clear as the treatment landscape for solid tumours is changing with the advent of targeted therapies.
Multiple studies have examined the association of various mutations and general clinicopathological features, in stage IIIc unresectable and stage IV melanoma patients with BRAF V600E mutations. They were younger at the time of diagnosis (median age, 44.7 years), had primary cutaneous melanoma of superficial spreading or nodular histology, originating in the trunk or extremities and lacked evidence of chronic sun damage. Patients with V600K mutation were older (median age, 60 years) and had primaries of head and neck origin. Patients with BRAF mutations had shorter disease free interval from the diagnosis of the primary tumour to the development of metastatic disease (8), however patients with V600E mutations had longer OS from stage IV diagnosis, in comparison with those with V600K mutations (34).
The NRAS mutations are less common in advanced melanoma. The most common NRAS mutations are in exon 2 (82%—Q61R, Q61K, Q61L) and less often in exon 1 (18%) (34). The exon 2 mutations are frequent in cutaneous primaries of the nodular subtype; while the exon 1 mutations are frequently associated with mucosal melanomas (34). Lack of available targeted therapy for this group of patients confers worse outcomes in comparison with BRAF mutated advanced melanoma patients on BRAF inhibitor therapy and this opens up avenues to explore new pharmacotherapies in this patient subgroup.
Mutations activating the receptor tyrosine kinase c-kit are uncommon in cutaneous (chronic sun damage) melanomas (3%), but occur commonly in melanomas arising from mucous membranes and acral skin (20%) (35). Asian patients had a higher incidence of c-kit mutations (10.8%) when compared to Caucasians patients (2-4%) (27,36,37). Although there are multiple other aberrations in melanoma, and other aberrations are gaining increasing importance e.g., NF1 mutations, the BRAF, NRAS and KIT mutations are mutually exclusive (except for approximately 1%), and at this stage remain the focus for the development of targeted drugs.
BRAF inhibitors and combination therapies in BRAF mutated melanoma brain metastases
Both dabrafenib and vemurafenib are potent small molecule reversible ATP-competitive serine threonine kinase inhibitor of BRAF V600E mutated melanoma cells, resulting in inhibition of downstream activated MAPK pathway and has substantial activity in BRAF mutated melanoma brain metastasis. Phase 3 trials of dabrafenib and vemurafenib showed significantly improved response rates, progression free and OS, in comparison to dacarbazine, in BRAF mutated advanced melanoma but excluded patients with active or untreated brain metastasis (38,39).
Activity of dabrafenib in active brain metastasis was demonstrated in a large multicentre, open-label, phase 2 trial which enrolled 172 BRAF V600E, V600K mutated metastatic melanoma patients and at least one measurable brain metastasis (5-40 mm diameter), into cohort A (n=89) with no previous local therapy and cohort B (n=83) with progression after local therapy or WBRT (3). The primary end point of overall intracranial objective response was 39% (29/74) in cohort A and 31% (20/65) in cohort B. The response rates were lower for V600K subgroup at 7% (1/15) and 22% (4/18) for cohorts A and B respectively, however numbers were very small. The median survival was 33 and 31 weeks for cohorts A and B respectively. In this study, dabrafenib was active despite progression after previous local therapy and was well tolerated, with adverse events consistent with previous studies. The numbers of spontaneous intracranial haemorrhages were lower with dabrafenib therapy (3).
Similarly there are studies and case reports of vemurafenib including a neoadjuvant approach enabling subsequent local therapies for lesions which were initially thought unresectable (40). A small prospective study of vemurafenib at 960 mg twice daily in 24 patients with BRAF V600 mutation positive with very poor prognosis and symptomatic unresectable melanoma brain metastasis (failing at least one previous therapy and requiring corticosteroids for symptom control) demonstrated activity (22). The median treatment duration was 3.8 (0.1-11.3) months, median PFS was 3.9 (95% CI, 3.0-5.5) months, median survival was 5.3 (95% CI, 3.9-6.6) months and overall partial response at both intracranial and extracranial sites was achieved in 10/24 (42%; 95% CI, 22.1-63.4) patients and stable disease in 9/24 (38%; 95% CI, 18.8-59.4) patients (22). A recent large phase 2 study of vemurafenib in 146 metastatic melanoma patients with active brain metastasis showed a similar objective response of 20% and a median PFS of 3.7 months (41). Retrospective case series have demonstrated activity of vemurafenib, supporting the results of the prospective studies (42).
Concordance of the BRAF mutation between metastases within an individual patient can vary by test used. In studies using immunohistochemistry, there was very high concordance between primary melanoma and multiple metastases, whereas studies using molecular tests varied (43,44). Despite this finding, the intracranial responses parallel extracranial responses (2,3,22). However acquired resistance to single agent BRAF inhibitors alone develops within 6-7 months of therapy (45) and is mainly driven by MAPK reactivation, via BRAF copy number gains, aberrant BRAF splicing, mutations in NRAS or MEK1/2 and upregulation of receptor tyrosine kinases (46-48). As predicted by preclinical studies, combination of a BRAF inhibitor and a MEK inhibitor, in comparison with BRAF inhibitor monotherapy, significantly improved response rates, median progression free/OS and decreased skin oncogenic toxicities, in phase 1/2 (n=162, median PFS 9.4 versus 5.8 months, RR 76% versus 54%) (45) and in phase 3 studies [COMBI-D (dabrafenib/trametinib versus dabrafenib): median PFS 9.3 versus 8.8 months (49), COBRIM (vemurafenib/cobimetinib versus vemurafenib): median PFS 9.9 versus 6.2 months] (50). These studies have not included patients with active brain metastasis and this is currently explored in several active phase 2 trials of single agent BRAF inhibitor (NCT01378975: vemurafenib), combination of BRAF/MEK inhibitor therapy (NCT02039947: COMBI-MB; NCT02230306: co-BRIM3) and of combination therapy in the neoadjuvant setting (NCT01978236) prior to resection of brain metastasis (Table 1) (51-53). Triplet combinations of BRAF/MEK inhibitors and pembrolizumab (NCT02130466) or other targeted drugs e.g., CDK 4/6 inhibitors (NCT02159066), are currently being explored in advanced melanoma without active brain metastases (51).
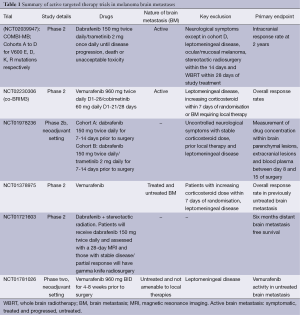
Full table
Several phase 2 studies of patients with V600 BRAF mutant metastatic melanoma, single agent trametinib or binimetinib demonstrated response rates of 17% to 25% in BRAF inhibitor naïve cohort (54-56) and a median progression-free survival (PFS) of 4.8 months compared with investigators choice chemotherapy (55), but has not been explored in brain metastases, and given its lesser activity than single agent BRAF inhibitor, not continued to be studied as a single agent.
MEK inhibitors and combination drug therapies in NRAS mutant melanoma
Several MEK inhibitors are in development in solid tumours, but the two most extensively investigated in melanoma are binimetinib (MEK162) and trametinib. MEK inhibitors have shown single agent activity in V600 BRAF mutated melanoma and NRAS mutated melanoma in patients without active brain metastases. In patients with NRAS mutated melanoma, single agent MEK 162, resulted in 11% (3/28 patients) partial response rates, 68% (19/28 patients) disease control rate and a PFS of 3.7 months (56). Phase 1/2 trials of combination of MEK inhibitors with a MDM2 inhibitor (AMG 232) (NCT02110355) and with a CDK 4/6 inhibitor (NCT02065063), amongst other combinations, are actively recruiting patients with NRAS mutated tumours, however active brain metastasis are excluded from these trials.
KIT inhibitors in c-kit mutant melanoma
There is currently no evidence of activity of c-kit inhibitors in patients with melanoma brain metastasis (57). The extracranial response rate to imatinib is approximately 30% with a median progression free survival of 3-4 months (58-60). The current management of c-kit mutation positive melanoma patients involves enrolment in clinical trials using c-kit inhibitor or immunotherapies or combination therapies targeting multiple independent pathways such MAPK, PI3K and immune mediated pathways.
Immunotherapy
Interleukin-2 (IL-2)
Historically high dose IL-2 has had a limited role in advanced melanoma in patients with good performance status and organ function (61-63) and no established role in treating brain metastasis. The need for hospitalisation and the propensity to cause serious deleterious side effects including multiorgan failure, fatal cerebral edema and severe neurotoxicity limits its use. A large retrospective study showed some evidence of activity in patients with previously treated brain metastasis with little benefit in the untreated cohort (64). However a recent retrospective review of eight patients with stable brain metastasis showed progressive disease in 7/8 patients, with several grade three events (65).
Checkpoint inhibitors
Ipilimumab
The fully humanised monoclonal antibody against cytotoxic T lymphocyte antigen-4 (CTLA4), ipilimumab, shows activity in melanoma brain metastasis, particularly if asymptomatic (23) and improves OS (66,67). A phase 2, multicentre, open-label study administered four doses of 10 mg/kg q3 weekly, followed by maintenance therapy, to 72 metastatic melanoma patients with brain metastasis; cohort A (n=51), asymptomatic patients and cohort B (n=21), symptomatic patients requiring corticosteroids (23). The global disease control was 18% [modified World Health Organisation (mWHO)] and 26% (immune-related response criteria-irRC) for cohort A and 5% (mWHO) and 10% (irRC) for cohort B. The median OS was 7 months for cohort A and 3.7 months for cohort B. Twelve patients (24%) in cohort A and two patients (10%) in cohort B achieved disease control within the brain. Interestingly, 33% (17/51) in cohort A and 24% (5/21) in cohort B, had prior WBRT. The most common adverse events were fatigue, diarrhoea, nausea, headache, rash and pruritis (23). A phase 2 study of ipilimumab and fotemustine showed an overall immune disease control rate of rate of 50% and a median progression free survival of 4.3 months, with increased incidence of both haematological and non-haematological toxicity (68). A phase 3 trial of this combination (EudraCT Number: 2012-004301-27, NIBIT-M2) is currently active (53).
PD1/PDL1 inhibitors and combinations
Long term exposure of T-cells to melanoma antigens leads to expression of programmed cell death 1 (PD-1) receptor (binds to its primary ligand PDL1 within the tumour microenvironment) and second ligand PDL2 by antigen presenting cells, resulting in negative regulation of the effector phase of T-cell responses against melanoma cells (69-71). A small study showed marked heterogeneity of PDL1 expression, by immunohistochemistry, between the primary and the metastasis and a positive correlation between PDL1 expression in locoregional metastasis and melanoma specific survival (72). PDL1 was expressed in about 47% of brain metastasis (72). Anti-PD1 antibody nivolumab and pembrolizumab have demonstrated highly durable response rates (RR 41% and 38% respectively), with minimal toxicity, in large phase 1 trials (73,74) and these were further confirmed in subsequent phase 3 trials of these agents versus chemotherapy in the first line (75) and in the second line setting after failure of anti-CTLA4 therapy (76). These agents in combination with ipilimumab are currently explored in several ongoing phase 2 trials in advanced melanoma patients with (NCT02374242, NCT02320058) and without (NCT01866319, NCT01721772) brain metastasis (51). A recent phase 1 trial of anti-PDL1 antibody (MPDL3280A) has shown activity in metastatic melanoma (77).
Anti-PD1 Brain Collaboration (ABC) (NCT02374242) is an Australian Brain randomised phase 2 trial exploring the activity of anti-PD1 antibody alone and in combination with ipilimumab, in melanoma brain metastasis (51). Eligible patients are those with histologically confirmed metastatic melanoma, measurable brain lesions (5-40 mm) and immunotherapy naïve. Refractoriness to prior BRAF therapy does not preclude participation in the trial. Six patients will initially be enrolled into cohort 1 (asymptomatic and previously untreated brain metastases) and cohort 2 (patients with previously treated brain metastases that have progressed after local treatment, and/or patients who have neurological symptoms related to brain metastases, and/or have leptomeningeal disease). Patients in cohort 1 and 2 will receive nivolumab 3 mg/kg, every 2 weeks. An interim safety analysis will be conducted when six patients from cohort 1 have received 6 weeks of therapy with nivolumab; to assess adverse events related to brain metastases, such as intracranial haemorrhage, seizure or other neurological toxicity. If these adverse events are acceptable (occur in ≤2 of the six patients at a CTCAE grade no higher than 2), then the study will be extended to include an additional cohort of 30 patients with asymptomatic and previously untreated brain metastases to a combination of nivolumab (1 mg/kg, every 2 weeks, for four doses, then continue 3 mg/kg every 2 weeks subsequently) and ipilimumab (3 mg/kg, every 3 weeks for four doses). The primary endpoint is the intracranial response rate (complete and partial response in intracranial metastases as measured using RECIST 1.1 criteria (modified for brain metastasis). Another phase 2 trial (NCT02320058) is also exploring the activity of combination of ipilimumab and nivolumab in active melanoma brain metastases (51).
Chemotherapy
Fotemustine and temozolomide have been trialled in melanoma brain metastasis but have shown disappointing results, limiting its role in clinical management in this setting. A phase 2 study of temozolomide (150 mg/m2/d ×5 days every 28 days) in 151 patients with previously untreated brain metastasis showed a response rate of 6% and a median OS of 3.2 months (17). A phase 3 trial of fotemustine versus dacarbazine, in 43 patients with melanoma brain metastasis, showed a brain response rate of only 5.3% for fotemustine and 0% for dacarbazine (78).
Local therapies for melanoma brain metastasis
The evidence for use of local therapies in treating melanoma brain metastasis is based on large retrospective melanoma specific studies or based on inference from non-melanoma studies (79,80). Surgery is useful in managing solitary or limited intracranial disease especially those with symptoms or complications such as mass effect or haemorrhage and there is some evidence of improved survival (79,81,82). Radiosurgery may be used for small asymptomatic non-haemorrhagic lesions (83,84), and reported 12 months local control rates ranges from 52% to 75% (83,85,86). The addition of SRS to WBRT (87,88) or surgery (89,90) improves local control and distant brain free survival, but does not affect OS. A phase 3 trial of WBRT following local treatment of melanoma brain metastasis is currently underway, testing distant intracranial failure, OS, neurocognitive function and quality of life (NCT01503827). The WBRT is generally reserved for diffuse or leptomeningeal disease and the combination of WBRT and targeted therapy vandetanib (EudraCT Number: 2011-0006661-12, sponsor protocol number: OCTO_022) and ipilimumab (EudraCT Number: 2013-001132-22, sponsor protocol number: GEM-1202) are currently being tested in phase 2 trials (53). The addition of WBRT to SRS may affect the quality of life through neurocognitive decline (91,92) and needs careful consideration while managing such patients.
Approach to management of metastatic melanoma with brain metastasis
The advent of new targeted therapies, immune modulating therapies and sandwiching local therapies with systemic therapies has changed the way melanoma brain metastasis are managed, and the prognosis for such patients. The treatment decisions, based on key factors such as mutation status, performance status, burden and pace of extracranial disease and active brain metastasis, should be made in consultation with a multidisciplinary team of neurosurgeons, medical oncologist, radiation oncologist and others involved in the patient’s care.
Participation in a clinical trial should be encouraged, if an appropriate trial is available. For BRAF mutated patients, depending on the key factors listed above, the systemic therapy of choice may be either a targeted BRAF inhibitor therapy, preferably in combination with a MEK inhibitor, or an immune check point inhibitor. Until there is data from the anti-PD1 brain trials, check point inhibitors should be given with or shortly after local brain therapy as the response rate in brain is not high. For BRAF wild-type patients, immune check point inhibitor therapy is the systemic therapy of choice, usually given concurrently with or shortly after local brain therapy. Although ipilimumab is recommended first line outside of a clinical trial, and anti-PD1 therapy is commenced only after progression on ipilimumab, this paradigm may change, and anti-PD1 therapy may become the first-line choice alone or in combination with ipilimumab. The slow onset of response and low response rate with ipilimumab needs careful monitoring and in instances of concerns of early disease progression, changing early to anti-PD1 therapy should be considered. A schematic representation of management of metastatic melanoma is outlined in Figure 2 (25).
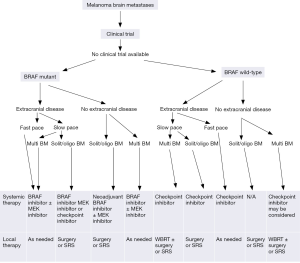
Key points
- Melanoma brain metastasis remains a major clinical problem and a multidisciplinary approach to management should be adopted.
- Enrolment into an appropriate clinical trial, when available, should be encouraged as the first step towards managing melanoma brain metastases.
- Not all patients with melanoma brain metastases require systemic therapy, e.g., solitary brain metastasis with absence of extracranial disease.
- BRAF inhibitors have shown a high rate of intracranial objective responses that parallel extracranial responses and should be considered in BRAF mutated melanoma especially in symptomatic patients with a high disease burden (Table 2).
- The focus for immune therapies has shifted to incorporate anti-PD1 antibodies. The FDA in the USA approved anti-PD1 therapy after ipilimumab progression, however results of phase 3 trials using first-line anti-PD1 antibodies or in combination with ipilimumab, will likely place anti-PD1 therapy as a backbone therapy, particularly for BRAF wild type melanoma with a high disease burden.
- In asymptomatic brain metastasis, not requiring steroid therapy, ipilimumab offers objective responses and survival benefit, similar to that seen with extracranial disease.
- Clinical trials of therapies specifically targeting NRAS or c-kit mutations in melanoma, have excluded patients with active brain metastases to date, largely because of the need to first determine combinations of therapies with good activity in extracranial metastases. As we determine optimal combinations to take forward into trials of patients with active brain metastases, these patients should be encouraged to participate in trials of immunotherapies, and trials for BRAF wildtype melanoma.
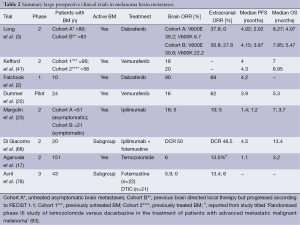
Full table
Conclusions
Brain metastasis is frequently encountered in clinical practice and while research has opened up several pathways of treatment, the prognosis remains guarded. Although our experience with targeted and immune therapies shows promising activity in melanoma brain metastases, clinical trial evidence of activity for many of these drugs, especially in combination, is currently in progress. Enrolment into clinical trials is essential, to develop evidenced-based practice paradigms for management of this difficult disease, to study mechanisms of intracranial response and resistance to treatment, and to devise better ways of treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Balch CM, Balch GC, Sharma RR. Identifying early melanomas at higher risk for metastases. J Clin Oncol 2012;30:1406-7. [PubMed]
- Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 2012;379:1893-901. [PubMed]
- Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:1087-95. [PubMed]
- Jakob JA, Bassett RL Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012;118:4014-23. [PubMed]
- Ramakrishna N, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma; local therapies for central nervous system metastases. Am Soc Clin Oncol Educ Book 2013;399-403. [PubMed]
- Bedikian AY, Wei C, Detry M, et al. Predictive factors for the development of brain metastasis in advanced unresectable metastatic melanoma. Am J Clin Oncol 2011;34:603-10. [PubMed]
- Schoenewolf NL, Belloni B, Simcock M, et al. Clinical implications of distinct metastasizing preferences of different melanoma subtypes. Eur J Dermatol 2014;24:236-41. [PubMed]
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 2011;29:1239-46. [PubMed]
- Ekedahl H, Cirenajwis H, Harbst K, et al. The clinical significance of BRAF and NRAS mutations in a clinic-based metastatic melanoma cohort. Br J Dermatol 2013;169:1049-55. [PubMed]
- Devitt B, Liu W, Salemi R, et al. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment Cell Melanoma Res 2011;24:666-72. [PubMed]
- Bucheit AD, Chen G, Siroy A, et al. Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAFV600 mutations. Clin Cancer Res 2014;20:5527-36. [PubMed]
- Eigentler TK, Figl A, Krex D, et al. Number of metastases, serum lactate dehydrogenase level, and type of treatment are prognostic factors in patients with brain metastases of malignant melanoma. Cancer 2011;117:1697-703. [PubMed]
- Morris SL, Low SH, A'Hern RP, et al. A prognostic index that predicts outcome following palliative whole brain radiotherapy for patients with metastatic malignant melanoma. Br J Cancer 2004;91:829-33. [PubMed]
- Marcus DM, Lowe M, Khan MK, et al. Prognostic factors for overall survival after radiosurgery for brain metastases from melanoma. Am J Clin Oncol 2014;37:580-4. [PubMed]
- Nieder C, Nestle U, Motaref B, et al. Prognostic factors in brain metastases: should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes? Int J Radiat Oncol Biol Phys 2000;46:297-302. [PubMed]
- Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011;117:1687-96. [PubMed]
- Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol 2004;22:2101-7. [PubMed]
- Atkins MB, Sosman JA, Agarwala S, et al. Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II Cytokine Working Group study. Cancer 2008;113:2139-45. [PubMed]
- Chang J, Atkinson H, A'Hern R, et al. A phase II study of the sequential administration of dacarbazine and fotemustine in the treatment of cerebral metastases from malignant melanoma. Eur J Cancer 1994;30A:2093-5. [PubMed]
- Daponte A, Signoriello S, Maiorino L, et al. Phase III randomized study of fotemustine and dacarbazine versus dacarbazine with or without interferon-α in advanced malignant melanoma. J Transl Med 2013;11:38. [PubMed]
- Mittapalli RK, Vaidhyanathan S, Dudek AZ, et al. Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther 2013;344:655-64. [PubMed]
- Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer 2014;50:611-21. [PubMed]
- Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012;13:459-65. [PubMed]
- Long GV, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma: the emerging role of systemic therapies. Am Soc Clin Oncol Educ Book 2013;393-8. [PubMed]
- Lyle M, Long GV. The role of systemic therapies in the management of melanoma brain metastases. Curr Opin Oncol 2014;26:222-9. [PubMed]
- Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell 2012;150:251-63. [PubMed]
- Siroy AE, Boland GM, Milton DR, et al. Beyond BRAF(V600): clinical mutation panel testing by next-generation sequencing in advanced melanoma. J Invest Dermatol 2015;135:508-15. [PubMed]
- Meckbach D, Keim U, Richter S, et al. BRAF-V600 mutations have no prognostic impact in stage IV melanoma patients treated with monochemotherapy. PLoS One 2014;9:e89218. [PubMed]
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev 2007;16:991-7. [PubMed]
- Ihle MA, Fassunke J, König K, et al. Comparison of high resolution melting analysis, pyrosequencing, next generation sequencing and immunohistochemistry to conventional Sanger sequencing for the detection of p.V600E and non-p.V600E BRAF mutations. BMC Cancer 2014;14:13. [PubMed]
- cobas® 4800 BRAF V600 Mutation Test. Available online: http://molecular.roche.com/assays/Pages/cobas4800BRAFV600MutationTest.aspx
- Long GV, Wilmott JS, Capper D, et al. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol 2013;37:61-5. [PubMed]
- Capper D, Preusser M, Habel A, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol 2011;122:11-9. [PubMed]
- Bucheit AD, Syklawer E, Jakob JA, et al. Clinical characteristics and outcomes with specific BRAF and NRAS mutations in patients with metastatic melanoma. Cancer 2013;119:3821-9. [PubMed]
- Curtin JA, Busam K, Pinkel D, et al. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 2006;24:4340-6. [PubMed]
- Kong Y, Si L, Zhu Y, et al. Large-scale analysis of KIT aberrations in Chinese patients with melanoma. Clin Cancer Res 2011;17:1684-91. [PubMed]
- Lyle M, Long GV. Diagnosis and treatment of KIT-mutant metastatic melanoma. J Clin Oncol 2013;31:3176-81. [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [PubMed]
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323-32. [PubMed]
- Melnik I, Lotem M, Yoffe B. A new role of vemurafenib as a neoadjuvant treatment of axillary and brain melanoma metastases. Case Rep Oncol Med 2013;2013:794239.
- Kefford RF, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicenter study. Pigment Cell Melanoma Res 2013;26:965.
- Dzienis MR, Atkinson VG. Response rate to vemurafenib in patients with B-RAF-positive melanoma brain metastases: a retrospective review. Melanoma Res 2014;24:349-53. [PubMed]
- Colombino M, Capone M, Lissia A, et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol 2012;30:2522-9. [PubMed]
- Menzies AM, Lum T, Wilmott JS, et al. Intrapatient homogeneity of BRAFV600E expression in melanoma. Am J Surg Pathol 2014;38:377-82. [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [PubMed]
- Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res 2014;20:1965-77. [PubMed]
- Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov 2014;4:94-109. [PubMed]
- Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014;4:80-93. [PubMed]
- Long GV, Stroyakovsky DL, Gogas H, et al. COMBI-d: A randomized, double-blinded, Phase III study comparing the combination of dabrafenib and trametinib to dabrafenib and trametinib placebo as first-line therapy in patients (pts) with unresectable or metastatic BRAFV600E/Kmutation-positive cutaneous melanoma. J Clin Oncol 2015;32:abstr 9011^.
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867-76. [PubMed]
- US National Institutes of Health. ClinicalTrials.gov. 2015. Available online: https://www.clinicaltrials.gov/
- Australian New Zealand Clinical Trials Registry. 2015. Available online: http://www.anzctr.org.au/
- EU Clinical Trials Register. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search
- Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 2013;31:482-9. [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [PubMed]
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013;14:249-56. [PubMed]
- Handolias D, Hamilton AL, Salemi R, et al. Clinical responses observed with imatinib or sorafenib in melanoma patients expressing mutations in KIT. Br J Cancer 2010;102:1219-23. [PubMed]
- Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011;305:2327-34. [PubMed]
- Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 2011;29:2904-9. [PubMed]
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol 2013;31:3182-90. [PubMed]
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. [PubMed]
- Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 2000;6 Suppl 1:S11-4. [PubMed]
- Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994;271:907-13. [PubMed]
- Guirguis LM, Yang JC, White DE, et al. Safety and efficacy of high-dose interleukin-2 therapy in patients with brain metastases. J Immunother 2002;25:82-7. [PubMed]
- Chu MB, Fesler MJ, Armbrecht ES, et al. High-Dose Interleukin-2 (HD IL-2) Therapy Should Be Considered for Treatment of Patients with Melanoma Brain Metastases. Chemother Res Pract 2013;2013:726925.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol 2012;13:879-86. [PubMed]
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887-95. [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [PubMed]
- Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006;203:883-95. [PubMed]
- Madore J, Vilain RE, Menzies AM, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res 2015;28:245-53. [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [PubMed]
- Weber JS, Minor DR, D'Angelo SP, et al. Phase 3 randomized, open-label study of nivolumab (anti-PD-1; BMS-936558, ONO-4538) versus investigator's choice chemotherapy (ICC) in patients with advanced melanoma after prior anti-CTLA4 therapy. Ann Oncol 2014;25:abstr LBA3.
- Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol 2013;31:abstr 9010.
- Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol 2004;22:1118-25. [PubMed]
- Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol 2004;22:1293-300. [PubMed]
- Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999;43:795-803. [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-9. [PubMed]
- Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 1994;29:711-7. [PubMed]
- Liew DN, Kano H, Kondziolka D, et al. Outcome predictors of Gamma Knife surgery for melanoma brain metastases. Clinical article. J Neurosurg 2011;114:769-79. [PubMed]
- Mathieu D, Kondziolka D, Cooper PB, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Clin Neurosurg 2007;54:241-7. [PubMed]
- Chang EL, Selek U, Hassenbusch SJ 3rd, et al. Outcome variation among "radioresistant" brain metastases treated with stereotactic radiosurgery. Neurosurgery 2005;56:936-45; discussion 936-45. [PubMed]
- Lwu S, Goetz P, Monsalves E, et al. Stereotactic radiosurgery for the treatment of melanoma and renal cell carcinoma brain metastases. Oncol Rep 2013;29:407-12. [PubMed]
- Samlowski WE, Watson GA, Wang M, et al. Multimodality treatment of melanoma brain metastases incorporating stereotactic radiosurgery (SRS). Cancer 2007;109:1855-62. [PubMed]
- Selek U, Chang EL, Hassenbusch SJ 3rd, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys 2004;59:1097-106. [PubMed]
- Soltys SG, Adler JR, Lipani JD, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys 2008;70:187-93. [PubMed]
- Jensen CA, Chan MD, McCoy TP, et al. Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg 2011;114:1585-91. [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [PubMed]
- Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000;18:158-66. [PubMed]