Chinese guidelines on the management of renal cell carcinoma (2015 edition)
IntroductionOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
The guideline was established by the kidney cancer panel under the Chinese Society of Clinical Oncology (CSCO) in accordance with the basic principles of evidence-based medicine (Table 1) and the clinical practices on renal cancer in China, based on the real conditions of China, and by referring to the USA NCCN Clinical Practice Guidelines in Oncology-Kidney Cancer (1) and the European Association of Urology (EAU) Guidelines on Renal Cell Carcinoma (2). The renal cancer referred in these guidelines is the renal cell carcinoma (RCC) and does not include various tumors originated from renal interstitium and renal pelvis/urothelial system.

Full table
Epidemiology and etiologyOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
RCC accounts for 2-3% of all adult malignancies. According to the GLOBOCAN data released in 2015 (3), the prevalence and mortality of RCC were 4.4/100,000 and 1.8/100,000 worldwide; they were 6.0/100,000 and 2.5/100,000 in males and 3.1/100,000 and 1.2/100,000 in females. Epidemiologically, the incidence of RCC is significantly correlated with region, race, gender, and age. It is higher in developed countries than in developing countries, especially in North America, Australia/New Zealand, and Europe (above 10/100,000 in males) and is relatively low in Africa and Pacific islands (lower than 1.5/100,000). It is higher in urban areas than in rural areas and in males than in females. In particular, the incidence of RCC is 10-20% higher in African Americans than in other ethnic groups.
The prevalence of RCC remains low in China. Generally, the incidence and mortality of RCC are remarkably higher in males than in females, with a male/female ratio of 2:1. In addition, they are higher in urban areas than in rural areas (4). According to the National Cancer Registry under the National Cancer Center of China (5), there were 45,096 new RCC cases in 2011, accounting for 1.34% of all malignancies; the incidence of RCC was 3.35/100,000, ranking 15th among all malignancies. The incidence of RCC was 4.38/100,000 and 2.26/100,000 in males and females and 4.73/100,000 and 1.89/100,000 in urban areas and rural areas. RCC can occur in all age groups; although it is relatively rare in individuals under 35 years old, the incidence of RCC rises rapidly in populations aged >35 years and reaches a peak (14.7/100,000) in the 75- to 80-year-old group. The mortality of RCC is 1.12/100,000 nationwide; more specifically, it is 1.43/100,000 in males and 0.81/100,000 in females and is 1.44/100,000 in urban areas and 0.79/100,000 in rural areas. The RCC accounts for 0.5% of all cancer deaths, ranking 16th among all cancers.
RCC can be divided into hereditary and sporadic RCC according to the presence/absence of family heredity. The hereditary RCC refers to kidney cancer with specific genetic changes and has a family trait, accounting for 2−4% of all kidney cancer. Most clinical diagnosed RCC cases are patients with sporadic RCC. Early in 1990s, studies on the molecular genetics of hereditary RCC revealed the molecular and biological mechanisms of some sporadic RCC cases; however, the exact etiologies of sporadic RCC remain unclear. Many epidemiological studies have identified some factors associated with the pathogenesis of RCC. The following four factors have been demonstrated to be associated with the RCC onset in evidence-based studies: (I) genetics (6); (II) smoking (7); (III) obesity (8); and (IV) hypertension and anti-hypertensive treatment (9).
DiagnosisOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
Symptoms
Patients with localized RCC typically have no obvious symptoms. The disease is often found during health check-ups or when receiving medical imaging for other reasons. Thus, there are more localized RCC patients without any symptom and less RCC patients with symptoms/signs. In particular, RCC patients with “triple symptoms” including bloody urine, pain, and swelling are in clinical settings. In 2010, the Chinese Urological Association analyzed the clinical data of 1,975 patients with initially diagnosed RCC in 23 medical centers from August 2007 to October 2008 and found that 62.7% of the patients did not have any clinical manifestation; rather, the disease was found and diagnosed during health check-ups or when receiving abdominal imaging (ultrasound, CT or MRI) (10). A diagnosis of RCC was made in the remaining 37.3% of patients who were presented due to certain clinical manifestations including low back pain (60.5%), bloody urine (45.6%), anemia (12.8%), hypertension (12.7%), wasting (11.8%), abnormal kidney function (9.1%), abnormal liver function (7.5%), mass (7.0%), fever (5.5%), abnormal platelet count (5.1%, and other manifestations (21.7%). Few patients had the typical clinical manifestations (pain, bloody urine, and mass) of RCC. Among these 1,975 patients, distant metastasis was found by intraoperative imaging in 8.9% of patients, and lymph node metastasis was confirmed by postoperative pathology in 6.4% of patients. In patients with metastatic RCC (mRCC), bone pain, fractures, severe anemia, cough, and hemoptysis can occur, depending on the metastasis site and disease severity. It has been reported that 10−40% of RCC patients may have paraneoplastic syndrome (11), which is clinically presented as hypertension, fever, anemia, weight loss, cachexia, polycythemia, liver function abnormalities, hypercalcemia, hyperglycemia, increased erythrocyte sedimentation rate (ESR), neuromuscular disease, amyloidosis, galactorrhea, and coagulation abnormalities.
Signs
No specific sign is seen in patients with early-stage RCC. Signs may occur in less than 10% of RCC patients. Abdominal mass may be detected in patients with huge RCC; swelling of the left supraclavicular lymph nodes may be found in patients with lymph node metastasis; lower extremity edema may occur in patients whose inferior vena cava tumor thrombosis severely blocks venous return; and varicocele at the left side that does not change with body position can be found in patients with renal vein thrombosis in left kidney.
Laboratory examinations
The main laboratory test items include renal function (serum urea nitrogen, creatinine, and glomerular filtration rate), liver function, serum calcium, serum glucose, ESR, and alkaline phosphatase, and lactate dehydrogenase; in addition, routine blood test, routine urinalysis, and test for blood coagulation should also be performed. Urine cytology should be performed for patients with renal tumors adjacent to or involving renal pelvis. Radionuclide renography should be performed in patients with solitary renal tumor, bilateral renal tumor, abnormal renal function indicators, and/or other disease (e.g., diabetes, chronic pyelonephritis, polycystic kidney disease, and contralateral kidney stones) that may damage renal function to learn the renal function.
Imaging examinations
The clinical diagnosis and staging of RCC is mainly based on the findings of medical imaging.
Abdominal ultrasound or color Doppler ultrasound
Abdominal ultrasound or color Doppler ultrasound is the simplest method for detecting renal tumor and is commonly applied in clinical settings. Contrast-enhanced renal ultrasound is useful for differentiating benign and malignant renal tumors and is feasible for the renal tumor patients with chronic renal failure or for patients who are not feasible for contrast-enhanced CT due to iodine allergy. Also, it can be applied for the differential diagnosis of complex kidney cyst.
Abdominal CT/MRI
Plain and contrast-enhanced abdominal CT is the main approach for the clinical diagnosis and staging of RCC. Thin-slice plain CT scan plus contrast-enhanced CT should be performed for the differential diagnosis of cystic disease. MRI has many advantages in the differential diagnosis of RCC and hemorrhagic renal cysts and in determining the range of venous tumor thrombus.
Chest X-ray
Chest X-ray at antero-posterior (AP) and lateral positions is the common approach for pre-operative examination and postoperative follow-up in RCC patients.
Other imaging examinations
For patients with clinically localized (T1-2 stage) RCC, chest CT, cerebral MRI/CT, radionuclide bone scan, and PET-CT are usually not required. These examination options may be considered in the following conditions:
- Indications of chest CT: (i) chest X-ray for suspected lesions; (ii) for patients with a clinical stage of > stage III (category 1);
- Indications of cerebral MRI/CT: patients with headache or other relevant neurological symptoms (category 1);
- Indications of radionuclide bone imaging: (i) with relevant bone symptoms; (ii) increased alkaline phosphatase; (iii) with a clinical stage of > stage III (category 1);
- Indications of PET/PET-CT: with a need to confirm whether there is any distant metastasis; or, for efficacy evaluation after systemic therapy.
Renal biopsy
Preoperative renal biopsy is not recommended for patients whose RCC has been confirmed by imaging and is feasible for surgery [(including radical renal resection and nephron-sparing surgery (NSS)]. For RCC patients who are not feasible for surgery (due to elderly age or with surgical contraindications) or patients with inoperable advanced RCC, renal biopsy can be performed before systemic therapy to obtain a definite pathologic diagnosis (including pathology type), which facilitates the selection of therapeutic drugs. In RCC patients who have chosen ablation therapy (e.g., radiofrequency ablation and cryoablation), renal biopsy should be performed to obtain pathological diagnosis before ablation (category 2A). For patients with a renal tumor whose malignancy is unknown after medical imaging, NSS or routine (every 1−3 months) imaging may be applied; renal biopsy is generally not recommended.
StagingOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
The AJCC Cancer Staging Manual (7th edition) [2010] is recommended for RCC staging (Tables 2,3) (12) (category 1).

Full table

Full table
PathologyOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
Hereditary RCC mainly occurs in young and mid-aged adults, with the lesions often being bilateral or multiple. In contrast, the sporadic RCC typically occurs in older populations, with the lesion being solitary at a single side. About 2−4% of patients with sporadic RCC, the tumor can successively or simultaneously invade both kidneys. RCC is mainly located at the upper or lower pole of kidney. The tumor size varies dramatically: it can range from 0.5−30 cm in the newly diagnosed patients, with a mean value of 5.4 cm (10).
Histological classification
According to the World Health Organization classification of tumors of the urinary system and male genital organs (2004 edition) (13), RCC includes the following 10 subtypes: clear-cell RCC, multilocular cystic RCC, papillary RCC (type I and type II), chromophobe RCC, collecting duct carcinoma (also known as Bellini tumors), medullary carcinoma, translocation RCC (mainly characterised by the translocation of XP11.2), RCC following the therapy of neuroblastoma tumors, mucinous tubular and spindle cell carcinoma, and unclassified RCC.
In addition, several new RCC types have been proposed: tubulocystic carcinoma (previously known as low-grade collecting duct carcinoma) (14,15), thyroid-like follicular carcinoma of the kidney (16), t[6,11] translocation RCC (17), clear-cell papillary RCC (18), and XP11.2 translocation renal cancer (19).
It is expected that the 4th edition of World Health Organization classification of tumors of the urinary system and male genital organs (20), which is to be released in 2016, will add new RCC subtypes including clear-cell papillary RCC, hereditary leiomyomatosis and renal cell cancer (HLRCC)-associated RCC, tubular cystic RCC, and acquired cystic disease-associated RCC. In addition, the multilocular cystic RCC is renamed as multilocular cystic renal cell neoplasms of low malignant potential, which can more accurately reflect its biological behaviors. The RCC following the therapy of neuroblastoma tumors will not be listed as an independent subtype; rather, it will be included into the “unclassified RCC”.
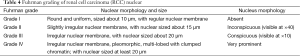
Histological grading
Fuhrman grading is the most common pathological grading system for RCC (based on the shapes and sizes of tumor cell nuclei and nucleoli) (21) (Table 4). RCC can be divided into four grades (grade I−II), and higher grade is associated with poorer prognosis. For RCC with sarcomatous change and rhabdomyoid differentiation, the Fuhrman grade of the tumor cell nucleus will be grade IV (the highest grade). Fuhrman grading of RCC is based on the region with the highest grade, which should be confirmed in two 400× visual fields. Fuhrman grading system is mainly applied for clear-cell RCC and papillary RCC but not for chromophobe RCC. A new grading standard has been proposed for chromophobe RCC (22); however, it has not been widely recognized and applied.
In 2013, the International Society of Urological Pathology (ISUP) proposed a new grading system (known as ISUP grading system or ISUP/WHO grading system) (23), which grades the tumors merely based on the change of tumor cell nucleoli. Both ISUP and WHO have recommended the use of ISUP grading system for RCC grading; however, this system has not been widely applied in China.
Full table
Standardization of RCC pathology report
Pathology report informs the treatment. Therefore, it must include all the required information. The content of a pathology report may differ according to the specimen name.
Biopsy specimens
Tumor type and Fuhrman grade of tumor cell nuclear (if applicable).
Partial nephrectomy specimens
Specimen name, surgical procedure, tumor type, tumor size, and tumor cell nuclear Fuhramn grade (if applicable); sarcomatoid change or rhabdomyoid differentiation (if any) and its proportion; tumor necrosis (if any) and its proportion; vascular tumor thrombus; invasion of the perirenal adipose tissue after the tumor breaks through the renal capsule; resection margin (renal parenchyma or perirenal adipose tissue); and pathological stage of tumor (pTN).
Radical nephrectomy (RN) specimens
Specimen name, surgical procedure, tumor type, tumor size, and tumor cell nuclear Fuhramn grade (if applicable); sarcomatoid change or rhabdomyoid differentiation (if any) and its proportion; tumor necrosis (if any) and its proportion; vascular tumor thrombus; invasion of major vessels (e.g., inferior vena cava and renal vein); invasion of adipose tissue of pelvis; invasion of the perirenal adipose tissue after the tumor breaks through the renal capsule; invasion of adrenal gland; resection margin (ureter, renal vein, inferior vena cava or perirenal adipose tissue); lymph node involvement; and pathological stage of tumor (pTN).
Prognostic factorsOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
The most important prognostic factor of RCC is the pathological stage. In addition, the histological grade, performance status, symptoms, tissue necrosis inside tumor, as well as abnormalities and changes in some biochemical indicators can also predict the outcomes of an RCC patient. Generally, the papillary RCC and chromophobe RCC have better prognosis than clear-cell RCC; the papillary RCC type I has better prognosis than papillary RCC type II; and the collecting tube RCC has poorer prognosis than clear-type RCC (24-26). However, a multicenter study on the prognoses of cell subtypes and prognosis of RCC patients showed that the outcomes of different RCC subtypes were not significantly different if they were in the same stage or grade (27).
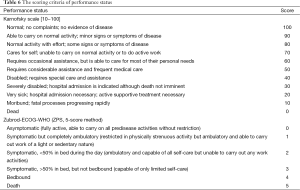
Currently, no well recognized and applicable prognosis evaluation system has been available for early- and intermediate-stage RCC. The risk factor scoring system for predicting the prognosis of patients with mRCC in the NCCN Clinical Practice Guidelines in Oncology is recommended (Table 5). The scoring criteria of the physical status see Table 6.

Full table
Full table
Surgical treatmentOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
The principles of surgical treatment are established according to cTNM stage, which is determined based on the findings of medical imaging. For early-stage RCC, surgery is the most important treatment. Early and appropriate surgery is critical to achieve good outcomes in RCC patients.
Surgical treatment of localized RCC and locally advanced RCC
Localized RCC refers to the T1-2N0M0 stage RCC in the AJCC TNM staging system [2010]; clinically it refers to stages I and II, also known as “early-stage RCC”. Among with the wide application of medical imaging technology and the implementation of health check-ups, localized RCC has accounted for over 50% among RCC patients. In contrast, the locally advanced RCC refers to RCC that has regional lymph node metastasis and/or inferior vena cava tumor thrombus and/or adrenal metastasis or if the tumor has invaded perirenal adipose tissue and/or renal sinus adipose tissue (but does not beyond Gerota’s fascia); however, it has no distant metastasis. It is classified as stage III disease in the 2010 edition of AJCC guidelines. Previously it is known as “locally end-stage RCC”. For patients with localized and locally advanced RCC, surgery remains the preferred treatment that is possible to cure the disease (category 1) (28-32). For patients with localized RCC, NSS may be performed (category 2A). Most retrospective literature has demonstrated that the rate of chronic kidney disease (CKD) was lower in patients who had undergone NSS than in those who had received RN (33-38). However, up to now the only randomized controlled clinical trial in this regard showed that these two surgical procedures had no significant difference in terms of survival (39). The feasible procedures including open surgeries, laparoscopic surgeries, and robotic surgeries. The surgery may be performed via abdominal or lower back approaches. However, no evidence has shown that these surgical procedures have significant difference in controlling RCC (40-45). For patients who are not feasible for laparoscopic NSS, open NSS is preferred. No evidence has shown that lymph node dissection (LND) can benefit the patients. Therefore, regional or extended LND is not recommended for patients with localized RCC. However, intraoperative lymph node removal is recommended for patients with suspected regional lymph node metastasis (indicated by pre-operative CT or found during intraoperative exploration), so as to facilitate postoperative pathological staging (46).
Radical nephrectomy (RN)
The scope of conventional RN includes perinephric fascia, perirenal fat, ipsilateral and/or contralateral adrenal glands, and regional adrenal lymph nodes. This concept has changed. Routine intraoperative adrenalectomy and lymphadenectomy are no longer recommended.
The indications of RN that spares the ipsilateral adrenal gland include (category 2A) (47,48): (I) with a clinical stage of I or II; (II) the tumor is located in the middle and lower parts of the kidney; and (III) preoperative CT shows normal adrenal gland. If abnormality in ipsilateral adrenal gland is detected during the surgery, the ipsilateral adrenal gland should be removed (49).
In a phase III randomized controlled trial conducted by European Organization for Research and Treatment of Cancer (EORTC) (46), a total of 772 patients with localized RCC were enrolled, with an attempt to evaluate the role of regional LND in the surgical treatment of localized RCC. The patients were randomized into RN group (n=389) and RN + LND group (n=383), with a median follow-up period of 12.6 years. The results showed that these two groups were not significantly different in terms of rate of complications, overall survival (OS), time to progress, and progression-free survival (PFS). Therefore, regional or extended LND is not recommended for patients with localized RCC (category 1). However, resection of abnormal lymph nodes is recommended for patients in whom preoperative CT shows swollen lymph nodes or intraoperative exploration shows abnormality, so as to obtain sufficient information for staging.
Nephron-sparing surgery (NSS)
- Indications of NSS (50,51): (i) RCC occurs in anatomic kidney or functionally isolated kidney; and (ii) bilateral RCC;
- Relative indications of NSS (50,51): in patients with one-sided RCC, the contralateral kidney has certain benign disease such as kidney stones, chronic pyelonephritis, or other diseases (e.g., hypertension, diabetes, renal artery stenosis, etc.) that may lead to worsening of renal function;
- Optional indications of NSS: solitary RCC in clinical stages T1a or T1b, with normal contralateral kidney function, and the tumor site makes the NSS technically feasible (1,2) (category 2A).
The indications and relative indications of NSS have no specific requirement on the size of RCC. The thickness of the normal renal parenchyma around the tumor to be resected is not a key issue only if a negative resection margin is finally ensured (category 2A) (2,52,53).
Management of RCC with inferior vena cava tumor thrombus
About 4−10% of RCC patients may also have inferior vena cava tumor thrombus, among which 55−70% can be cured by RN combined with inferior vena cava tumor thrombus removal. The tumor thrombus is mostly staged according to the Mayo classification of macroscopic venous invasion in RCC (54) (Table 7). Preoperative MRI (or contrast-enhanced CT) is recommended to clarify the scope of tumor thrombus involvement before establishing a treatment protocol. The need for cardiopulmonary bypass or resection of segments of inferior vena cava should be decided according to the scope of tumor thrombus and degree of infiltration. This complex surgery is associated with high perioperative complication rates and case-fatality. Therefore, a multidisciplinary team with rich experiences is needed to perform the surgery.

Full table
Renal artery embolization
Some studies have shown that preoperative renal artery embolization is not obviously helpful for prolonging survival, reducing intraoperative bleeding, and preventing postoperative complications (55,56).
Ablation therapy
Ablation therapies including radio-frequency ablation (RFA), cryoablation, and high-intensity focused ultrasound (HIFU) can be applied in inoperable small RCC patients; however, the specific procedure should be chosen in strict accordance with the indications (category 2B).
The indications of ablation therapy include: (I) not suitable for open surgery or laparoscopic surgery; (II) the nephron function needs to be spared as possible; (III) contraindicated for general anesthesia; (IV) renal insufficiency; (V) tumor sized <4 cm and peripherally located (57-59).
Observation of follow-up
For small RCC patients with short life expectancy, old age, and multiple complications, close observation and follow-up is also a reasonable option (category 2B) (60-63).
Surgical treatment of metabolic RCC
Indications of cytoreductive nephrectomy
Cytoreductive nephrectomy is preferred for patients with good physical status and low risk factors (see Table 5) (category 1) (64,65).
Indications of palliative nephrectomy
Palliative nephrectomy is recommended for resolving/improving symptoms such as severe hematuria and pain in RCC patients.
Surgical treatment of metastatic lesions
Surgical treatment can be applied in patients who developed solitary metastasis following RN and in RCC patients with solitary metastasis and good physical status. For patients who have developed metastasis, removal of the metastatic lesion and kidney surgery can be performed simultaneously or in different phases, depending on the physical status of the patients (66).
Medical treatmentOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
Surgery remains the main treatment for localized kidney cancer. After accurate pathologic staging (pTNM) according to the postoperative pathology, the postoperative treatment protocol (including the use of postoperative adjuvant therapy) can be established accordingly. For advanced RCC, especially mRCC, multidisciplinary management including medical treatment is needed to prolong survival and maximize quality of life.
Postoperative adjuvant therapy
Tumor recurrence will occur in 20−30% of localized RCC patients. In most patients the recurrence occurs within 30 years, with a median time to recurrence of 1−2 years. Randomized controlled clinical trials showed that assisted cytokine therapy [interferon-α (IFN-α) and interleukin-2 (IL-2)], radiotherapy, and chemotherapy did not reduce the recurrence and metastasis rates in RCC patients (67). Administration of autogenous tumor vaccine also showed no benefit to the patients. Anti-angiogenesis targeted drugs have shown good efficacies in treating mRCC; however, no evidence has demonstrated that postoperative administration of these drugs can also benefit patients with localized or locally advanced RCC (68). Close observation and follow-up remains the recommended protocol for patients with localized or locally advanced RCC. Patients at high risk of metastasis/recurrence may be encouraged to participate in clinical trials.
Medical treatment of mRCC
Since sorafenib was approved for the treatment of mRCC in 2005, the management of mRCC has entered an era of targeted therapy. The United States Food and Drug Administration (FDA) has approved 7 kinds of targeted drugs; based on their action mechanisms, these drugs can be divided into anti-VEGF/VEGFR drugs (e.g., sorafenib, sunitinib, and pazopanib, and axitinib) and mTOR-inhibitors (e.g., everolimus and temsirolimus). Up to now the China Food and Drug Administration (CFDA) has approved sorafenib, sunitinib, everolimus and axitinib for the treatment of mRCC. Compared with cytokine therapies, these targeted drugs remarkably improve the efficacy and prolong the survival time of patients. In recent years, immunotherapies, represented by immune checkpoint inhibitors, have developed rapidly. Relevant clinical trials are on the way. In future, such immunotherapies may be applied for the treatment of advanced RCC.
First-line treatment of clear-cell RCC
Targeted therapy
Molecularly targeted therapy is the preferred first-line treatment for clear-cell RCC. Research has found that the majority of clear-cell RCC cells are featured by VHL gene deletion or inactivation, which induces the up-regulation of HIF gene and thus leads to the over-expressions of PDGF, VEGF, and CalX genes. These biological mechanisms may shed light on the molecularly targeted therapy of clear-cell RCC.
Sunitinib
Sunitinib is a multitargeted receptor tyrosine kinase inhibitor (TKI), with its main action targets including vascular endothelial growth factor receptors 1−3 (VEGFR 1−3), platelet-derived growth factor receptors (PDGFR-α and PDGFR-β), stem cell growth factor receptors (c-KIT), and FMS-like tyrosine kinase 3 (FLT-3). It can fight against tumor angiogenesis and inhibit tumor cell proliferation (69).
An international randomized phase III clinical trial compared the efficacy and safety of sunitinib with those of IFN-α in the first-line treatment of mRCC and found that the sunitinib group had an objective response rate of 31%, median PFS of 11 months, and median OS of 26.4 months, which were significantly superior to those in the control (IFN-α) group (70,71). A phase IV multicenter study of the efficacy and safety of sunitinib as first-line therapy in Chinese patients with mRCC showed that the objective response rate was 31.1% (PFS, 14.2 months; median OS, 30.7 months) (72).
Based on the above evidences, The CSCO expert committee recommends that sunitinib can be used in the first-line treatment for advanced clear-cell RCC. Usage: 50 mg qd, po, administered on a 4/2 (4-week on, 2-week off) schedule (category 1). Since the 4/2 schedule has a relatively high rate of adverse reactions, some authors have proposed to use a 2/1 administration schedule (2-week on, 1-week off), which had a higher tolerance and unaffacted efficacy (73). The 2/1 sunitinib administration schedule has also been attempted in a domestic study (74), showing similar results (category 2B).
Sorafenib
Sorafenib is the first licensed multitargeted receptor tyrosine inhibitor for mRCC, with dual anti-tumor effects: on one hand, it can inhibit tumor growth by inhibiting the RAF/MEK/ERK signaling pathway; on the other hand, it inhibits tumor growth by exerting effects on targets including VEGFR, PDGFR, c-KIT, FLT-3, and MET (75).
An international randomized phase II clinical trial on the use of sorafenib in the first-line treatment of mRCC showed that the median PFS was 5.7 months, which was comparable with interferon therapy (76). Subsequently, in an international multicenter phase III clinical trial on the use of sorafenib as a control for first-line treatment of mRCC (TIV0-1) showed that the objective response rate of sorafenib was 24% (median PFS, 9.1 months; median OS, 29.3 months) (77).
Totally 62 patients were enrolled in the registration trial of sorafenib in China, which showed the objective response rate was 19.4% (disease control rate, 77.4%; median PFS, 9.6 months) (78). Subsequent clinical practices as well as retrospective analyses further confirmed the effectiveness of sorafenib in the first-line treatment for mRCC (79). In the largest multicenter retrospective analysis of patients with advanced RCC (n=845) in China compared the efficacy between sorafenib and sunitinib and found that the median PFS was 11.1 and 10.0 months in these two groups, respectively, showing significant difference; the median OS was 24.0 months in both groups, showing no significant difference (80).
Based on the above evidences, The CSCO expert committee recommends that sorafenib can be used in the first-line treatment for advanced clear-cell RCC. Usage: 400 mg bid (category 2A).
Pazopanib
Pazopanib is also a multitargeted receptor TKI, with its main action targets including VEGFR1-3, PDGFRot-P, and c-KIT (81).
An international multicenter phase III trial on the use of pazopanib for mRCC showed that the median PFS was 11.1 months and the objective response rate was 30%, which was significantly superior to those in the placebo group; the final survival analysis showed that the median OS was 22.6 months (82,83). In the COMPARZ study (84), which compared pazopanib vs. sunitinib in the treatment of locally advanced and/or mRCC and participated by many Chinese centers, the median PFS of pazopanib and sunitinib was 10.5 vs. 10.2 months, the ORR was 33% vs. 29%, the median OS was 28.4 vs. 29.3 months; thus, the efficacy of pazopanib is non-inferior to that of sunitinib, and the quality of life is superior in pazopanib than in sunitinib group. This study enrolled 367 Asian patients (including Chinese subjects); subgroup analysis of the pazopanib group showed that the median PFS was 8.4 months in Asian patients, which was not significantly different from those in European and American populations (85).
Based on the above evidences, the CSCO expert committee recommends that pazopanib can be used in the first-line treatment for advanced clear-cell RCC. Usage: 800 mg qd (category 1).
Bevacizumab + IFN-α
Bevacizumab is an anti-VEGF monoclonal antibody. When combined with IFN-α, it can be used in the first-line treatment for mRCC. The phase III data from AVOREN study and CALGB90206 study have demonstrated its clinical efficacy. In the AVOREN study, the combination of bevacizumab and IFN-α, when used in the first-line treatment, achieved a median PFS of 10.2 months, an objective response rate of 30.6%, and a median OS of 23.3 months, which were all superior to those in IFN-α alone group (86). The large-scale CALGB 90206 study also obtained the similar results (87). The indications of bevacizumab approved in China are advanced colorectal cancer and non-small cell lung cancer. No clinical evidence has been available for the use of bevacizumab for RCC.
Based on the above clinical data, the CSCO kidney cancer panel recommends that bevacizumab + IFN-α can be used in the first-line treatment for advanced clear-cell RCC (category 1). Usage: bevacizumab: 10 mg/kg q2w; IFN-α: 9 MIU tiw.
Temsirolimus
Temsirolimus (CCI-779) is an inhibitor of mammalian target of rapamycin protein (mTOR). In addition to its inhibitory effect on mTOR signal, it can also inhibit angiogenesis, which is achieved by suppressing the transcription of hypoxia-inducible factor 1 (HIF-1) and reducing the stimulation on vascular growth factors such as VEGF, PDGF, and TGF (88,89).
In the ARCC study (an international multicenter randomized controlled phase II trial on the use of temsirolimus in first-line treatment of patients with mRCC) (90), the temsirolimus monotherapy achieved a median OS of 10.9 months and a median PFS of 5.5 months in high-risk patients, showing superior efficacy to IFN-α. A non-randomized, single-arm, open-label, phase II study conducted in Asian populations showed that, among 82 patients with mRCC, temsirolimus achieved a clinical benefit rate of 48%, objective response rate of 11%, and a median PFS of 7.3 months (91).
Although temsirolimus has not been licensed in China, the CSCO kidney cancer panel still recommends that temsirolimus can be used in the first-line treatment of high-risk patients with advanced clear-cell RR (category 1). Usage: 25 mg qw, till progression.
Axitinib
Axitinib is a novel multitargeted receptor TKI. A phase III trial showed that the median PFS was 10.1 months in axitinib group, which was superior to sorafenib group; however, the difference was not statistically significant (92). Subgroup analysis showed that the superiority of axitinib was more notable in patients who had received nephrectomy and patients with an ECOG score of 0.
Few Chinese literature has reported the use of axitinib in the first-line treatment of mRCC patients. Therefore, based on foreign clinical data, the CSCO kidney cancer panel recommends that axitinib can be used in the first-line treatment of the selected patients with advanced clear-cell RCC (category 2B). Usage: 5 mg bid.
Cytokine therapy
Before the introduction of targeted drugs, intermediate- and high-dose IFN-α or IL-2 remains the standard first-line treatment for metastatic clear-cell RCC, with an objective response rate of about 15%. However, since high-dose IL-2 treatment can be accompanied with obvious side effects, no IL-2 agent was manufactured in China. Thus, the cytokine therapy for metastatic clear-cell RCC is mainly based on IFN-α.
Interferon-α (IFN-α)
Many clinical trials have demonstrated that intermediate- and high-dose (>9 million IU) IFN-α can double the PFS in mRCC patients (compared with placebo) (93), especially in clear-cell RCC patients with a prognostic score of “low risk” or “intermediate risk”. Although the combination of IFN-α and IL-2 can increase the treatment effectiveness in mRCC patients, the PFS was not significantly different in IFN-α + IL-2 group and IFN-α monotherapy group.
Based on the specific situations in China, the CSCO kidney cancer panel recommends that intermediate- and high-dose IFN-α can be an optional treatment for metastatic clear-cell RCC patients who are not feasible to receive targeted drugs (category 1). Usage: IFN-α, 9 MIU tiw, for 12 weeks.
Interleukin-2 (IL-2)
In 1992, the U. S. FDA approved the use of high-dose IL-2 for the treatment of mRCC. It had an objective response rate of 15-25% and a complete remission rate of 3−5%. The treatment efficacy could last a long period of time, and the patient’s survivals were improved (94). However, intravenous injection of high-dose IL-2 could be associated with high rates of severe adverse reactions. Therefore, this administration route has no longer been widely used. Subcutaneous drug delivery and reduction in dosage can increase the treatment tolerance without remarkably changing the efficacy (95-97).
In a domestic trial on the subcutaneous injection of recombinant human IL-2 (Proleukin) for treating mRCC (98), the objective response rate of 19.4%, rate of stable disease was, 44.4%, median PFS was 6 months, and median OS was 22.5 months. Severe adverse reactions (grade 3) were rare, and the patients mainly had grade 1/2 (mild or moderate) adverse reactions in multiple systems.
Based on the above evidences, the CSCO kidney cancer panel recommends that high-dose IL-2 (Proleukin) can be used in metastatic clear-cell RCC patients who have good general conditions and normal heart and lung functions (category 2A). Usage: 18 MIU/d, IH, 5 d/w × 1w, 9 MIU q12h d1−2, 9 MIU qd d3−5 × 3w; repeated after one week of rest.
Second-line treatment of clear-cell RCC
Second-line treatment after failure of targeted drug therapy
Everolimus
Everolimus is an orally administered mTOR inhibitor. In an international multicenter randomized phase III clinical study (RECORD-1 study) (99,100), everolimus or placebo was applied in mRCC patients in whom the previous targeted drug therapy had failed; it was found that everolimus group had a significantly longer median PFS (4.9 months) than placebo group, with a clinical benefit rate of 64% and a median OS of 14.8 months. Notably, among patients whose condition failed to respond to sorafenib and sunitinib first-line treatment, the median PFS reached 5.4 months in the everolimus second-line treatment group, and the risk of disease progression decreased by 69% (99).
In the registration trial of everolimus in China (L2101 study), everolimus can be used in the second-line treatment after failure of TKI treatment, with a disease control rate of 61%, median PFS of 6.9 months, clinical benefit rate of 66%, 1-year survival rate of 56%, and 1-year PFS rate of 36% (101). According to the results of RECORD-4, an international multicenter clinical study on the use of everolimus in the second-line treatment of advanced RCC, released in the 2015 annual meeting of American Society of Clinical Oncology (ASCO), the median PFS of everolimus second-line treatment reached 7.8 months.
Based on the results of the above trials, the CSCO kidney cancer panel recommends that everolimus can be used in the second-line treatment of mRCC after failure of TKI treatment (category 1). Usage: 10 mg qd.
Axitinib
According to the findings of AXIS study, an international multicenter randomized phase III clinical trial comparing axitinib and sorafenib in treating mRCC that had progressed after cytokine or TKI treatment, axitinib had a significantly longer median PFS (6.7 months), along with an objective response rate of 19% and a median OS of 20.1 months (102,103). Stratified analysis showed, in patients who had previously received sunitinib first-line treatment, axitinib group had significantly longer median PFS than sorafenib group (4.8 vs. 3.4 months).
In a registration clinical trial on the use of axitinib in the second-line treatment of mRCC in Asian populations (mainly Chinese patients) (104) using similar design as the AXIS study, the median PFS was 6.5 and 4.8 months in axitinib group and sorafenib group, respectively, and the objective response rate was 23.7% and 10.1%, respectively. Subgroup analysis showed that the axitinib second-line treatment could achieve a median PFS of 4.7 months in patients who had previously received sunitinib therapy.
Based on the above evidences, the CSCO kidney cancer panel recommends that axitinib can be used in the second-line treatment of mRCC (category 1). Usage: 5 mg bid.
Increased dose of sorafenib
An international multicenter phase II trial on sorafenib first-line treatment explored the effectiveness and safety of increasing the dose of sorafenib after disease progression (76). After disease progression was observed in patients who had received sorafenib treatment at a standard dosage, the sorafenib dose was increased to 600 mg bid; after that the median PFS was 3.6 months, with tolerable adverse reactions. Some other studies on the increased dose of sorafenib in second-line treatment (including 800 mg bid) also obtained the similar outcomes (105-107).
Based on the results of these trials, the CSCO kidney cancer panel recommends that increased dose of sorabenib can be used in the second-line treatment of mRCC after failure of previous sorafinib treatment at standard dosage (category 2B). Usage: 600 mg qd, which may be gradually increased to 800 mg bid.
Other TKI-targeted drugs
In two recent prospective clinical studies on second-line targeted therapies (INTORSECT study and AXIS study), sorafenib was used as the control group. All the patients enrolled in the INTORSECT study were those who failed in the sunitinib treatment, among whom sorafenib used in second-line treatment achieved a median PFS of 3.9 months and a median OS of 16.6 months (108). In the AXIS study, sorafenib as second line treatment had a median PFS of 4.7 months and a median OS of 19.2 months; among them patients who had failed in previous sunitinib therapy had achieved a median PFS of 3.4 months (102,103).
In the SWITCH study, sequential sunitinib treatment after disease progression despite sorafenib treatment achieved a median PFS of 5.4 months (109). In another phase II trial, targeted therapy with sunitinib in the second-line treatment after disease progression despite bevacizumab treatment achieved an objective response rate of 23% and a median PFS of 30 weeks (110).
In a phase II clinical trial, pazopanib was applied in metastatic clear-cell RCC patients whose condition failed to respond to the previous first-line sunitinib or bevacizumab; the results showed that objective response rate was 27%, stable disease rate was 49%, median PFS was 7.5 months, and 24-month survival rate was 43% (111). In another retrospective study, use of pazopanib in second-line treatment achieved an objective response rate of 43% and a median PFS of 11 months (112).
Based on the results of the above clinical trials, the CSCO kidney cancer panel recommends that sorafenib can be used in the second-line treatment for disease progression despite sunitinib treatment (category 2A), sunitinib can be used in the second-line treatment for disease progression despite sorafenib treatment (category 2A), and pazopanib can be used in the second-line treatment for mRCC (category 2B),
Combinations of targeted drugs
In patients with advanced RCC, targeted drugs can act on different targets to achieve the anti-angiogenesis effect. Combinations of these targeted drugs can exert synergistic antitumor effectiveness (113). Some foreign studies (e.g., BEST study) have confirmed the safety and feasibility of the different combinations of targeted drugs (114).
Lenvatinib is a novel receptor TKI, with the main action targets including VEGFR1−3, fibroblast growth factor receptor 1−4 (FGFR1−4), PDGFR-α, RET, and KIT (115). A phase II trial compared the values of lenvatinib + everolimus, lenvatinib alone, or everolimus alone in treating mRCC that had progressed despite anti-VEGF treatment (116); the results showed that the combination group had a median PFS of 14.6 months and a median OS of 25.5 months, which were significantly superior to those in two control groups.
A domestic phase II trial applied sorafenib + bevacizumab in the second-line targeted treatment of advanced RCC (117); of 23 patients who failed in the first-line treatment with TKI, the overall objective response rate of 13.0%, the disease control rate was 69.6%, and the median PFS was 7.0 months. The grade 3/4 toxicities were mainly hand/foot/skin reactions and diarrhea.
Based on the results of the above trials, the CSCO kidney cancer panel recommends that sorafenib + bevacizumab can be used in the second-line treatment for mRCC patients after failure of TKI treatment (category 2B). Usage: Sorafenib: 400 mg bid; bevacizumab: 5 mg/kg q2w.
Second-line treatment after failure of cytokine therapy
Anti-angiogenesis TKIs may be useful in the subsequent treatment after the failure of first-line cytokine therapy in mRCC patients. In a phase III randomized clinical trial that applied sorafenib in metastatic clear-cell RCC patients after the failure of first-line cytokine therapy showed that sorafenib achieved a median PFS of 5.9 months, which was significantly longer than that in placebo group; meanwhile, the median OS was 19.3 months (118). In a phase II trial, sunitinib was used in the second-line treatment for mRCC patients after failure of first-line cytokine therapy; the results showed that the response rate was 45%, median PFS was 8.4 months, and median OS was 23.9 months (119,120). Subgroup analysis of an international multicenter phase III trial on the use of pazopanib for mRCC showed that the pazopanib group had significantly longer median PFS (7.4 months) than the placebo group (82,83).
Subgroup analysis of a phase III trial on the use of axitinib, a novel receptor TKI, in the second-line treatment of mRCC showed that, in patients who had received first-line cytokine therapy, axitinib achieved a median PFS of 12.1 months, which was significantly lower than that in control group (102,103).
Based on the results of the above trials, the CSCO kidney cancer panel recommends that sorafenib, sunitinib, pazopanib, and axitinib can be used in the second-line treatment for mRCC patients after failure of cytokine therapy (category 1).
Immunotherapy
With the increased understanding of the interactions between the immune system and cancer, novel immunotherapies gradually emerge. Immunological checkpoint inhibitors, in particular the anti-PD-1/anti-PD-L1 monoclonal antibodies, may be used for the treatment of advanced RCC in future (121,122).
A series of clinical studies have been conducted on the use of anti-PD-1 monoclonal antibody for the treatment of mRCC (122,123). In a phase II clinical study on the treatment of mRCC using nivolumab (124), all the subjects previously had received first-line treatment. The results showed that the objective response rate in the optimal dosing group (2 mg/kg) reached 22%; although the median PFS was only 4 months, the median OS, which was also the main research endpoint, reached 25.2 months. Currently, a phase III trial was comparing the efficacies of nivolumab and everolimus in treating advanced RCC when used in the second-line treatment.
Third-line treatment of clear-cell RCC
Sorafenib
In a phase III trial on the third-line targeted therapy of mRCC (GOLD study) (125), patients with metastatic clear-cell RCC whose condition progressed despite first-line treatment with sunitinib and second-line treatment with everolimus received third-line treatment with sorafenib; the results showed that the median PFS was 3.6 months, median OS was 11 months. This was the only phase III trial that had evaluated the role of multi-target TKI in the third-line treatment of mRCC. Based on the results of the above trials, the CSCO kidney cancer panel recommend that sorafenib can be used in the third-line treatment of mRCC (category 2B).
Everolimus
For low- and intermediate-risk patients, a subgroup analysis of the RECORD-1 study found that, in patients who had received sunitinib and sorafenib treatment, the third-line treatment with everolimus achieved a median PFS of 3.78 months, which was significantly superior to that in the placebo group. Therefore, everolimus can be used in the third-line treatment for patients who had failed in the first- and second-line treatment with vascular endothelial growth factor receptor tyrosine kinase inhibitor (VEGFR-TKI) (category 2A) (99,100).
Combinations of targeted drugs
Combinations of targeted drugs can also be attempted in the third-line treatment of advanced RCC. Sorafenib + bevacizumab and lenvatinib + everolimus can be useful options.
A multicente phase II trial conducted in China enrolled mRCC patients who had successively received sunitinib and everolimus; in the third-line treatment, these patients randomly received sorafenib + bevacizumab or sorafenib monotherapy. The preliminary results showed that the median PFS was 6.5 and 3.5 months in these two group, indicating sorafenib + bevacizumab may have better effectiveness (126).
Immunotherapy
In advanced RCC patients whose condition has failed to respond to first- and second-line targeted therapy, anti-PD-1 and anti-PD-L1 monoclonal antibodies may represent potential directions for future treatment.
Management of non-clear-cell RCC
Due to the small sample size, few large-scale randomized controlled clinical trials have explored the management of advanced clear-cell RCC. Extended clinical trials on sunitinib, sorafenib, and everolimus and small-sample phase II clinical trials have shown that these targeted drugs were effective in treating non-clear-cell RCC; however, there efficacies were inferior to those in clear-cell RCC (127-130).
Tyrosine kinase inhibitors (TKIs)
A randomized phase II Study of Afinitor (RAD001) vs. Sutent (Sunitinib) in Patients with Metastatic Non-Clear Cell Renal Cell Carcinoma (ASPEN) (131) showed that sunitinib treatment improved the patients’ survival: the median PFS was 8.3 months in sunitinib group and 5.6 months in everolimus group; and the median OS was 31.5 and 13.2 months, respectively. Based on the above clinical data, the CSCO kidney cancer panel recommends the use of sunitinib in the first-line treatment of non-clear-cell RCC (category 2B).
A multicenter phase II clinical study in China explored the combinations of sorafenib with gemcitabine and cisplatin in the first-line treatment for advanced collecting tube carcinoma (132) and found that the objective response rate was 33.3% and the median PFS was 10.0 months. Based on the above clinical data, the CSCO kidney cancer panel recommend the use of the combinations of sorafenib with gemcitabine and cisplatin in the first-line treatment of advanced collecting tube RCC (category 2B). Sorafenib can also be used in the first-line treatment for other types of non-clear-cell RCC (category 3).
mTOR inhibitors
Targeting at patients with a prognostic score of “high risk”, the ARCC study (a phase III clinical trial on temsirolimus) enrolled 72 patients with non-clear-cell RCC, and the results showed that temsirolimus treatment was superior to IFN-α treatment. Based on the above clinical data, the CSCO kidney cancer panel recommend the use of temsirolimus for the treatment of high-risk non-clear-cell RCC (category 2A).
The prognosis is poor in RCC patients with sarcomatoid differentiation. Chemotherapy alone or in combination with targeted therapy can be considered in patients whose condition fails to respond to targeted therapy or cytokine therapy (114,133). The useful chemotherapy drugs may include gemcitabine and doxorubicin (category 3) (115-137).
Management of toxicities associated with targeted therapy
mRCC is mainly treated with targeted therapy, whose toxicities are different from those induced by cytotoxic agents. Although all the targeted drugs for RCC are anti-angiogenesis inhibitors, their specific action targets differ, resulting in diversities in relevant toxicities. All toxicities can be assessed using the Common Terminology Criteria for Adverse Events (CTCAE) released by the National Cancer Institute.
Hand-foot skin reactions (HFSR)
HFSR are the most common adverse reactions of targeted drugs. They are mainly seen in patients who have received receptor TKIs such as sorafenib, sunitinib, pazopanib, and axitinib, with an incidence of 20-30%. They are manifested as a series of symptoms (e.g., skin tenderness and paresthesias as well as erythema, blisters, hyperkeratosis, dry skin, and skin blisters under induration) that affect feet and hands. A domestic study has found that the prophylactic use of urea ointment could lower the HFSR induced by sorabenib (138).
Targeted therapy may continue if grade 1/2 HFSR occurs; meanwhile, symptomatic treatment can be applied. For instance, topical use of urea ointment and strengthened skin care can effectively reduce or control the symptoms without discontinuing or reducing the drugs. However, for patients with grade 3 HFSR, dosage reduction or drug discontinuation may be needed. After the symptoms are improved, the therapy may be continued at a lower dose level.
Hypertension and other cardiovascular reactions
Targeted drugs for RCC may induce adverse cardiovascular reactions including hypertension, decreased left ventricular ejection fraction (LVEF), myocardial ischemia or myocardial infarction (Ml), and prolonged Q-T interval. Among them hypertension is most common, whereas other toxic reactions are less seen. Hypertension often occurs 1−2 weeks after medication and generally can persist along with drug administration. In most cases it can be well controlled by routine anti-hypertensive drugs. However, dosage reduction or drug discontinuation is required for uncontrollable hypertension. Thus, the blood pressure must be closely monitored during targeted therapy. Once blood pressure increases, drug treatment should be actively provided. Angiotensin-converting enzyme inhibitors are recommended.
Although myocardial ischemia and prolonged QT interval are less common, they are more severe and sometimes can be life-threatening. Therefore, an adequate safety assessment should be arranged for patients with a history of cardiovascular disease before TKI treatment.
Bone marrow suppression
Among the anti-angiogenesis targeted drugs, sunitinib has a remarkable inhibitory effect on bone marrow, which can be manifested by grade 3/4 thrombocytopenia. Although the incidence of grade 3/4 thrombocytopenia is low in Caucasian populations, it can be up to 21.9−29% in Asian populations (72,85). Therefore, the hemogram should be closely monitored during the sunitinib treatment. Once grade 3/4 thrombocytopenia occurs, the sunitinib treatment should be discontinued. Sunitinib treatment at a lower dose level may be considered after the bone marrow function is restored.
Interstitial pneumonia
Interstitial pneumonia is manifested as the non-infectious, non-malignant pulmonary infiltration. It typically occurs 2-6 months after the initial treatment. This condition may be asymptomatic or associated with non-specific respiratory symptoms (cough or difficulty breathing). Typically it is mild to moderate (and reversible), but may become severe and even fatal in a few cases. In a phase III clinical trial on everolimus, the incidence of non-infectious pneumonia was 14% (99,100). In a registration trial conducted in China (L2101 study), the incidence of interstitial pneumonia was 22% (101). Thus, adequate attention should be paid to this condition. Once interstitial pneumonia occurs, the everolimus treatment should be discontinued immediately. The patient may be administered with glucocorticoids. After the symptom is resolved, everolimus treatment may be re-initiated from the lowest dose level. If interstitial pneumonia recurs, everolimus treatment should be withdrawn permanently.
Everolimus treatment must be avoided in patients with a history of severe chronic obstructive pulmonary disease or severe pulmonary fibrosis.
Selection of targeted drugs
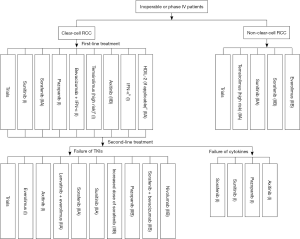
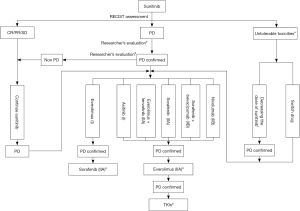
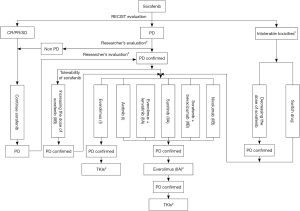
A variety of targeted drugs have been developed for the treatment of mRCC. Individualized treatment should be provided according to the tumor’s histological type, prognostic score, and individual conditions of patients to optimize the treatment. Targeted drugs can be selected according to Figure 1. For most Chinese patients, since there is limited access to licensed targeted drugs, the currently available drugs should be reasonably used (e.g., sequential administration; combinations) (Figures 2,3).
Treatment of metastases in special sites
Bone metastasis of RCC
Bone is a common metastatic site in RCC patients. The main sites include spine, pelvis, and proximal limb bones. The main symptoms include progressive pain intensification and dysfunction in the affected site. X-ray may display the presence of osteolytic bone destruction; thus, the metastatic site can easily develop pathologic fracture, which may even compress the spinal cord and cause paralysis. These patients should be treated mainly with targeted drugs, in combination with multidisciplinary treatment strategies such as surgery, radiotherapy, and bone protection. In patients with solitary metastatic lesion or if the lesion invades the weight-bearing bone, surgery may be performed to remove the metastatic lesion. In patients with weight-bearing bone metastasis at high risk of bone fracture may receive prophylactic internal fixation to avoid the occurrence of bone-related events. In patients who have developed pathological fractures or spinal cord compression-related symptoms, surgical treatment is preferred if the patient meets the following three conditions: (I) with an expected survival duration of more than 3 months; (II) with good physical status; and (III) the surgery can improve the patient’s quality of life and facilitates the subsequent targeted therapy, radiotherapy, and nursing. Percutaneous vertebroplasty can be used for the treatment of osteolytic spinal damage and pathological vertebral collapse. It can increase the hardness and pressure at the metastatic site and relieve local pain (139). Local low-dose palliative radiotherapy is effective in alleviating metastatic bone pain. In addition, active use of bone protectants drugs including bisphosphonates and denosumab is useful to reduce the occurence of bone-related events (140).
Brain metastasis of RCC
Radiotherapy has better effectiveness than surgical treatment in the management of brain metastases. In addition, radiotherapy is useful for multiple brain metastases; when in combination with dexamethasone and dehydrating agent, it can remarkably shrink tumor and peritumoral edema zone and thus alleviate symptoms associated with intracranial hypertension and other neurological symptoms. For patients with good physical status and simple brain metastasis (the brain metastatic sites are no more than 3 and the largest diameter of brain metastatic lesion is less than 3 cm), stereotactic radiotherapy (gamma knife, X knife, three-dimensional conformal radiotherapy, and intensity-modulated conformal radiotherapy) or brain surgery combined with radiotherapy is preferred. For patients with multiple brain metastases (the brain metastatic sites are more than 3 and the largest diameter of brain metastatic lesion is larger than 3 cm), whole-brain radiotherapy may be considered. Then, systemic anti-tumor medical treatment may be applied based on the patient’s tolerance (141,142). It has been reported that small-molecule targeted drugs can pass through the blood-brain barrier (143,144). Extended clinical trials on sunitinib and sorafenib have shown that these targeted drugs had certain efficacies in treating the brain metastasis of RCC (145,146).
Liver metastasis of RCC
The prognosis of patients with liver metastasis of RCC is extremely poor. Systemic targeted drug treatment should be considered firstly. In addition, local treatment including ablation therapy, hepatic artery perfusion/embolization chemotherapy, and precise radiotherapy may also be applied to enhance the local control of the metastatic lesion.
Follow-upOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
The routine follow-up includes: (I) history-taking; (II) physical examination; (III) laboratory tests, including routine urine test, urine blood test, serum urea nitrogen, creatinine, glomerular filtration rate, lactate dehydrogenase, liver function, alkaline phosphatase, and calcium; bone scan should be performed if the alkaline phosphatase abnormally increases and (or) has bone metastasis-associated symptoms; (IV) chest X-ray (AP and lateral positions); if the economic condition allows, chest CT is preferred; (V) RCC patients with acute nervous system signs or symptoms should immediately undergo cross-sectional CT or MRI scans of nervous system or spinal cord scan based on the symptoms associated with specific segments.
For stage pT1N0/ NxM0 RCC patients who had undergone surgical treatment, abdominal CT or MRI should be performed 3−12 months after the surgery; with the result as the baseline film, abdominal ultrasound, CT, or MRT should be performed every year and chest X-ray should be performed every year for three consecutive years to confirm whether there is lung metastasis. For stage pT2-4N0/NxM0 RCC patients who had undergone surgical treatment, the frequency of medical imaging should be changed to every 6 months for at least three consecutive years, and then every year till the fifth year (147).
AcknowledgementsOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
None.
FootnoteOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
Conflicts of Interest: The authors have no conflicts of interest to declare.
Updates in 2015 edition (from the 2013 edition)Other Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
EpidemiologyOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
The global prevalence of kidney cancer was updated (based on the Globocan data 2012).
The prevalence of kidney cancer in China was updated (based on the data from National Tumor Prevention and Control Research Office).
PathologyOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
The changes in the WHO classification method of renal caner (2016 edition) were added.
Surgical treatmentOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
The results of a randomized controlled trial comparing the nephron-sparing surgery and radical resection of kidney cancer were added.
Medical treatmentOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
- In the section of adjuvant therapy, a new statement was added as follows: for localized and locally advanced RCC, no evidence has demonstrated that post-operative anti-angiogenesis targeted drugs can benefit the patients;
- In the section of medical treatment of metabolic RCC:
- First-line treatment of clear-cell RCC
- Sunitinib 2/1 protocol was added;
- The data from a large-scale retrospective analysis of the efficacy of sorafenib in China were added;
- A new recommendation that axitinib can be used in the first-line treatment of mRCC was added;
- Second-line treatment of clear-cell RCC
- Clinical data concerning the use of axitinib in Asian patients were added;
- The evidence category on the increased dose of sorafenib was changed to 2B;
- The evidence category on the use of pazopanib in the second-line treatment was changed to 2B;
- The clinical research on lenvatinib plus everolimus for advanced RCC was added;
- The use of immune checkpoint inhibitors in the second-line treatment of advanced RCC was added.
- Third-line treatment of clear-cell RCC
- The recommendation of sorafenib in the third-line treatment was added;
- The evidence category on the use of everolimus in the third-line treatment was changed to 2A;
- The combinations of targeted drugs as well as the use of immunotherapy in third-line treatment were added.
- Treatment of non-clear-cell RCC
- The recommendation on the use of sunitinib in the first-line treatment of non-clear-cell RCC was added;
- The recommendation on the use of sorafenib combined with gemcitabine/cisplatin in the first-line treatment of collecting duct carcinoma of kidney was added;
- The evidence category on the use of temsirolimus in the treatment of non-clear-cell RCC was changed to 2A.
ReferencesOther Section
- Introduction
- Epidemiology and etiology
- Diagnosis
- Staging
- Pathology
- Prognostic factors
- Surgical treatment
- Medical treatment
- Follow-up
- Acknowledgements
- Footnote
- Updates in 2015 edition (from the 2013 edition)
- Epidemiology
- Pathology
- Surgical treatment
- Medical treatment
- References
- Mozter RJ, Jonasch E, Agarwal N, et al. NCCN Clinical Practice Guidelines in Oncology™ Kidney Cancer –V.3.2015.
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913-24. [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [PubMed]
- He J, Chen WQ. Chinese Cancer Registry Annual Report (2015). Beijing: Military Medical Science Press, 2012:93-6.
- Zhang SW, Han SJ, Zheng RS, et al. Kidney cancer incidence and mortality in China, 2011. Chinese National Cancer Center, 2015.
- Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 1993;260:1317-20. [PubMed]
- Hunt JD, van der Hel OL, McMillan GP, et al. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer 2005;114:101-8. [PubMed]
- Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer 2006;118:728-38. [PubMed]
- Dhote R, Thiounn N, Debré B, et al. Risk factors for adult renal cell carcinoma. Urol Clin North Am 2004;31:237-47. [PubMed]
- Li M, He ZS, Gao JP, et al. Multicenter analysis of clinical features of renal cancer. Chinese Journal of Urology 2010;31:77-80.
- Palapattu GS, Kristo B, Rajfer J. Paraneoplastic syndromes in urologic malignancy: the many faces of renal cell carcinoma. Rev Urol 2002;4:163-70. [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York: Springer Verlag, 2010:479-89.
- Ebele JN, Sauter G, Epstein JI, et al. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC, 2004:12-43.
- MacLennan GT, Farrow GM, Bostwick DG. Low-grade collecting duct carcinoma of the kidney: report of 13 cases of low-grade mucinous tubulocystic renal carcinoma of possible collecting duct origin. Urology 1997;50:679-84. [PubMed]
- Yang XJ, Zhou M, Hes O, et al. Tubulocystic carcinoma of the kidney: clinicopathologic and molecular characterization. Am J Surg Pathol 2008;32:177-87. [PubMed]
- Jung SJ, Chung JI, Park SH, et al. Thyroid follicular carcinoma-like tumor of kidney: a case report with morphologic, immunohistochemical, and genetic analysis. Am J Surg Pathol 2006;30:411-5. [PubMed]
- Argani P, Hawkins A, Griffin CA, et al. A distinctive pediatric renal neoplasm characterized by epithelioid morphology, basement membrane production, focal HMB45 immunoreactivity, and t(6;11)(p21.1;q12) chromosome translocation. Am J Pathol 2001;158:2089-96. [PubMed]
- Gobbo S, Eble JN, Martignoni G, et al. Clear cell papillary renal cell carcinoma: a distinct histopathological and molecular genetic entity. Am J Surg Pathol 2008;32:1239-45. [PubMed]
- Argani P, Aulmann S, Karanjawala Z, et al. Melanotic Xp11 translocation renal cancers: a distinctive neoplasm with overlapping features of PEComa, carcinoma, and melanoma. Am J Surg Pathol 2009;33:609-19. [PubMed]
- Moch H, Humphrey P, Ulbright T, et al. WHO Classifications of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC 2016. [Epub ahead of print].
- Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982;6:655-63. [PubMed]
- Paner GP, Amin MB, Alvarado-Cabrero I, et al. A novel tumor grading scheme for chromophobe renal cell carcinoma: prognostic utility and comparison with Fuhrman nuclear grade. Am J Surg Pathol 2010;34:1233-40. [PubMed]
- Delahunt B, Cheville JC, Martignomi G, et al. The International Society of Urological Pathology (ISUP) Grading System for Renal Cell Carcinoma and Other Prognostic Parameters. Am J Surg Pathol 2013;37:1490-504. [PubMed]
- Moch H, Gasser T, Amin MB, et al. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer 2000;89:604-14. [PubMed]
- Amin MB, Tamboli P, Javidan J, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol 2002;26:281-91. [PubMed]
- Motzer RJ, Bacik J, Mariani T, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol 2002;20:2376-81. [PubMed]
- Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: A multicenter experience. J Clin Oncol 2005;23:2763-71. [PubMed]
- Godley PA, Stinchcombe TE. Renal cell carcinoma. Curr Opin Oncol 1999;11:213-7. [PubMed]
- Pan BN, Xu RF, Guo X, et al. Clinical analysis of 525 patients with renal cancer. Chinese Journal of Urology 2000;21:135-7.
- Li Q, Cheng JY, Wang ZS, et al. Clinical analysis of 369 patients with renal cancer. Chinese Journal of Urology 2001;22:496-9.
- Paul R, Mordhorst J, Busch R, et al. Adrenal sparing surgery during radical nephrectomy in patients with renal cell cancer: a new algorithm. J Urol 2001;166:59-62. [PubMed]
- Yin CJ, Sui YG, Wu HF, et al. Radical treatment of renal cancer: report of 326 cases. Chinese Journal of Urology 2002;23:392-4.
- Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort stydy. Lancet Oncol 2006;7:735-40. [PubMed]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. [PubMed]
- Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephtectomy for pT1a renal cell masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol 2008;179:468-71. [PubMed]
- Weight CJ, Lieser G, Larson BT, et al. Partial nephrectomy is associated with improved overall survival compared to radical nephrectomy in patients with unanticipated benign renal tumours. Eur Urol 2010;58:293-8. [PubMed]
- Weight CJ, Larson BT, Gao T, et al. Elective partial nephrectomy in patients with clinical T1b renal tumors is associated with improved overall survival. Urology 2010;76:631-7. [PubMed]
- Kim SP, Thompson RH, Boorjian SA, et al. Comparative effectiveness for survival and renal function of partialand radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol 2012;188:51-7. [PubMed]
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011;59:543-52. [PubMed]
- Hemal AK, Kumar A, Kumar R, et al. Laparoscopic versus open radical nephrectomy for large renal tumors: a long-term prospective comparison. J Urol 2007;177:862-6. [PubMed]
- Hemal AK, Kumar A. A prospective comparison of laparoscopic and robotic radical nephrectomy for T1-2N0M0 renal cell carcinoma. World J Urol 2009;27:89-94. [PubMed]
- Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 2007;178:41-6. [PubMed]
- Lane BR, Gill IS. 7-year oncological outcomes after laparoscopic and open partial nephrectomy. J Urol 2010;183:473-9. [PubMed]
- Gong EM, Orvieto MA, Zorn KC, et al. Comparison of laparoscopic and open partial nephrectomy in clinical T1a renal tumors. J Endourol 2008;22:953-7. [PubMed]
- Marszalek M, Meixl H, Polajnar M, et al. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 Patients. Eur Urol 2009;55:1171-8. [PubMed]
- Blom JH, Van Poppel H, Maréchal JM, et al. Radical Nephrectomy with and without Lymph-Node Dissection: Final Results of European Organization for Research and Treatment of Cancer (EORTC) Randomized Phase 3 Trial 30881. Eur Urol 2009;55:28-34. [PubMed]
- Niu ZH, Xu CX, Wang JY, et al. Is ipsilateral adrenalectomy mandatory in radical nephrectomy for renal cell carrcinoa. Chinese Journal of Urology 1998;19:161-3.
- Wunderlich H, Schlichter A, Reichelt O, et al. Renal indications for adrenalectomy in renal cell carcinoma. Eur Urol 1999;35:272-6. [PubMed]
- Novick AC, Streem S, Montie JE, et al. Conservative surgery for renal cell carcinoma: a single-center experience with 100 patients. J Urol 1989;141:835-9. [PubMed]
- Gu FL. Renal cancer. In: Wu Jie-ping: Wu Jie-ping’s Urology. Jinan: Shandong Science & Technology Publishing House, 2004:889-917.
- Wein AJ, Kavoussi LR, Novick AC, et al. Campbell-Walsh Urology. 9 edition. Translated by Guo YL, Zhou LQ. Beijing: Peking University Medical Press, 2009:1651-733.
- Sutherland SE, Resnick MI, Maclennan GT, et al. Does the size of the surgical margin in partial nephrectomy for renal cell cancer really matter. J Urol 2002;167:61-4. [PubMed]
- Li QL, Guan HW, Zhang LZ, et al. ptimal margin in nephron-sparing surgery for renal cell carcinoma 4 cm or less in diameter. Chinese Journal of Surgery 2003;53:81-3.
- Blute ML, Leibovich BC, Lochse CM, et al. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int 2004;94:33-41. [PubMed]
- May M, Brookman-Amissah S, Pflanz S, et al. Pre-operative renal arterial embolisation does not provide survival benefit in patients with radical nephrectomy for renal cell carcinoma. Br J Radiol 2009;82:724-31. [PubMed]
- Subramanian VS, Stephenson AJ, Goldfarb DA, et al. Utility of preoperative renal artery embolization for management of renal tumors with inferior vena caval thrombi. Urology 2009;74:154-9. [PubMed]
- Ankem MK, Nakada SY. Needle-ablative nephron-sparing surgery. BJU Int 2005;95:46-51. [PubMed]
- Rabinovitch RA, Zelefsky MJ, Gaynor JJ, et al. Patterns of failure following surgical resection of renal cell carcinoma: Implications for adjuvant local and systemic therapy. J Clin Oncol 1994;12:206-12. [PubMed]
- Baird AD, Woolfenden KA, Desmond AD, et al. Outcome and survival with nonsurgical management of renal cell carcinoma. BJU Int 2003;91:600-2. [PubMed]
- Jewett MA, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 2011;60:39-44. [PubMed]
- Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer 2012;118:997-1006. [PubMed]
- Crispen PL, Viterbo R, Boorjian SA, et al. Natural history, growth kinetics, and outcomes of untreated clinically localized renal tumors under active surveillance. Cancer 2009;115:2844-52. [PubMed]
- Rosales JC, Haramis G, Moreno J, et al. Active surveillance for renal cortical neoplasms. J Urol 2010;183:1698-702. [PubMed]
- Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 2004;22:454-63. [PubMed]
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer. A combined analysis. J Urol 2004;171:1071-6. [PubMed]
- Kavolius JP, Mastorakos DP, Pavlovich C, et al. Resection of metastatic renal cell carcinoma. J Clin Oncol 1998;16:2261-6. [PubMed]
- Smaldone MC, Fung C, Uzzo RG, et al. Adjuvant and neoadjuvant therapies in high-risk renal cell carcinoma. Hematol Oncol Clin North Am 2011;25:765-91. [PubMed]
- Haas NB, Manola J, Uzzo RG, et al. Initial results from ASSURE (E2805): Adjuvant sorafenib or sunitinib for unfavorable renal carcinoma, an ECOG-ACRIN-led, NCTN phase III trial. J Clin Oncol 2015;33:abstr 403.
- Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU 11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platetel-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 2006;24:16-24. [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24. [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584-90. [PubMed]
- Qin SK. Sutent used in the first-line treatment of metastatic renal cell cancer in Chinese patients: a single-arm, open-label, prospective multicenter phase IV clinical trial. Beijing: Proceedings of the 15th National Conference on Clinical Oncology & 2012 Annual Meeting of CSCO.
- Najjar YG, Mittal K, Elson P, et al. A 2 weeks on and 1 week off schedule of sunitinib is associated with decreased toxicity in metastatic renal cell carcinoma. Eur J Cancer 2014;50:1084-89. [PubMed]
- Cui CL, Li SM, Chi ZH, et al. A pilot study of sunitinib as first.line therapy for metastatic renal cell carcinoma on a 2weeks on/1week off intermittent dosing schedule. Zhonghua Zhong Liu Za Zhi 2015;37:375-8. [PubMed]
- Wilhelm SL, Carter C, Tang L, et al. BAY-43-9006 exhibits btoad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [PubMed]
- Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:1280-9. [PubMed]
- Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 2013;31:3791-9. [PubMed]
- Zhou AP, He ZS, Yu SY, et al. Clinical trial on the use of sorafinib for metastatic renal cancer. Chinese Journal of Urology 2009;30:10-4.
- Guo F, Han T, Liu Z, et al. Prognostic analysis of Chinese patients with metastasis renal cell cancer receiving sorafenib: results from a multicenter long-term follow-up retrospective study. Onco Targets Ther 2015;8:1581-8. [PubMed]
- Sheng XN, Zhang HL, Li XS, et al. Retrospective analysis of multicenter first-line targeted therapies for advanced renal cancer. Xiamen: Proceedings of the 18th National Conference on Clinical Oncology & 2015 Annual Meeting of CSCO.
- Sonpavde G, Hutson TE, Sternberg CN. Pazopanib, a potent orally administered small-molecule multitargeted tyrosine kinase inhibitor for renal cell carcinoma. Expert Opin Investig Drugs 2008;17:253-61. [PubMed]
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061-8. [PubMed]
- Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer 2013;49:1287-96. [PubMed]
- Motzer R, Hutson T, Reeves J, et al. Randomized, open label, phase III trial of pazopanib versus sunitinib in first-line treatment of patients with metastatic renal cell carcinoma (mRCC); Results of the COMPARZ trial[abstract]. ESMO 2012:Abstract LBA8.
- Guo J, Jin J, Huang YR, et al. Comparison of PFS and safety for Asian compared to North American and European populations in the phase III trial of pazopanib versus sunitinib in patients with treatment-naive RCC (COMPARZ). J Clin Oncol 2013;31:abstr 366.
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007;370:2103-11. [PubMed]
- Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 2010;28:2137-43. [PubMed]
- Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 2005;307:1098-101. [PubMed]
- Hudson CC, Liu M, Chiang GG, et al. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol Cell Biol 2002;22:7004-14. [PubMed]
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81. [PubMed]
- Sun Y, Rha S, Lee SH, et al. Phase II study of the safety and efficacy of temsirolimus in East Asian patients with advanced renal cell carcinoma. Jpn J Clin Oncol 2012;42:836-44. [PubMed]
- Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013;14:1287-94. [PubMed]
- Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289-96. [PubMed]
- Bukowski RM. Natural history and therapy of metastatic renal cell carcinoma: the role of interleukin-2. Cancer 1997;80:1198-220. [PubMed]
- Rosenberg SA, Yang JC, White DE, et al. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg 1998;228:307-19. [PubMed]
- Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003;21:3127-32. [PubMed]
- Geertsen PF, Gore ME, Negrier S, et al. Safety and efficacy of subcutaneous and continuous intravenous infusion rIL-2 in patients with metastatic renal cell carcinoma. Br J Cancer 2004;90:1156-62. [PubMed]
- Sheng XN, Li JL, Guo J, et al. Clinical study on recombinant human interleukin-2 (Proleukin) in the treatment of metastatic renal cell carcinoma. Zhonghua Zhong Liu Za Zhi 2008;30:129-33. [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomized, placebo-controlled phase III trial. Lancet 2008;372:449-56. [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256-65. [PubMed]
- Guo J, Huang Y, Zhang X, et al. Safety and efficacy of everolimus in Chinese patients with metastatic renal cell carcinoma resistant to vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapy: an open-label phase 1b study. BMC Cancer 2013;13:136. [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomized phase 3 trial. Lancet 2011;378:1931-9. [PubMed]
- Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552-62. [PubMed]
- Qin S, Bi F, Jin J, et al. Axitinib versus sorafenib as a second-line therapy in Asian patients with metastatic renal cell carcinoma: results from a randomized registrational study. Onco Targets Ther 2015;8:1363-73. [PubMed]
- Amato R, Zhai J, Willis J, et al. A phase II trial of intrapatient dose-escalated sorafenib in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer 2012;10:153-8. [PubMed]
- Mancuso A, Di Paola ED, Leone A, et al. Phase II escalation study of sorafenib in patients with metastatic renal cell carcinoma who have been previously treated with anti-angiogenic treatment. BJU Int 2012;109:200-6. [PubMed]
- Wang HK, Zhang HL, Zhu Y, et al. A Phase II trial of dosage escalation of sorafenib in Asian patients with metastatic renal cell carcinoma. Future Oncol 2014;10:1941-51. [PubMed]
- Hutson TE, Escudier B, Esteban E, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 2014;32:760-7. [PubMed]
- Eichelberg C, Vervenne WL, De Santis M, et al. Switch: a randomised, sequential, open-label study to evaluate the efficacy and safety of sorafenib-sunitinib versus sunitinib-sorafenib in the treatment of metastatic renal cell cancer. Eur Urol 2015;68:837-47. [PubMed]
- Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 2008;26:3743-8. [PubMed]
- Hainsworth JD, Rubin MS, Arrowsmith ER, et al. Pazopanib as second-line treatment after sunitinib or bevacizumab in patients with advanced renal cell carcinoma: a Sarah Cannon Oncology Research Consortium Phase II Trial. Clin Genitourin Cancer 2013;11:270-5. [PubMed]
- Rautiola J, Utriainen T, Peltola K, et al. Pazopanib after sunitinib failure in patients with metastatic renal cell carcinoma. Acta Oncol 2014;53:113-8. [PubMed]
- Sosman JA, Puzanov I, Atkins MB. Opportunities and Obstacles to Combination Targeted Therapy in Renal Cell Cancer. Clin Cancer Res 2007;13:764s-769s. [PubMed]
- McDermott DF, Manola J, Pins M, et al. The BEST trial (E2804): A randomized phase II study of VEGF, RAF kinase, and mTOR combination targeted therapy (CTT) with bevacizumab (bev), sorafenib (sor), and temsirolimus (tem) in advanced renal cell carcinoma (RCC). J Clin Oncol 2013;31:abstr 345.
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621-30. [PubMed]
- Motzer RJ, Hutson TE, Glen H, et al. Randomized phase II, three-arm trial of lenvatinib (LEN), everolimus (EVE), and LEN+EVE in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2015;33:abstr 4506.
- Sheng XN, Chi ZH, Cui CL, et al. A phase II clinical study of sorafenib combined with bevacizumab in the second line targeted treatment of metastatic renal cell carcinoma. Chinese Journal of New Drugs 2014;23:317-20.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. [PubMed]
- Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006;295:2516-24. [PubMed]
- Motzer RJ, Rini BI, Michaelson MD, et al. Phase 2 trials of SU11248 show antitumor activity in second-line therapy for patients with metastatic renal cell carcinoma. J Clin Oncol 2005;23:abstr 4508.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Pal SK, Hu A, Chang M, et al. Programmed death-1 inhibition in renal cell carcinoma: clinical insights and future directions. Clin Adv Hematol Oncol 2014;12:90-9. [PubMed]
- Cho DC, Sosman JA, Sznol M. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2013;31:abstr 4505.
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol 2015;33:1430-7. [PubMed]
- Motzer RJ, Porta C, Vogelzang NJ, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:286-96. [PubMed]
- Guo J, Sheng X, Chi Z, et al. A randomized, open-label, multi-center phase II study to compare bevacizumab plus sorafenib versus sorafenib for the third-line treatment of patients with metastatic renal cell carcinoma (NCT02330783). J Clin Oncol 2015;33:abstr e15591.
- Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol 2008;26:127-31. [PubMed]
- Stadler WM, Figlin RA, McDermott DF, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer 2010;116:1272-80. [PubMed]
- Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol 2012;62:1013-9. [PubMed]
- Escudier B, Bracarda S, Maroto JP, et al. Open-label phase II trial of first-line everolimus monotherapy in patients with advanced papillary renal cell carcinoma:RAPTOR interim analysis [abstract]. ESMO 2012;abstr 2696.
- Armstrong AJ, Broderick S, Eisen T, et al. Final clinical results of a randomized phase II international trial of everolimus vs. sunitinib in patients with metastatic non-clear cell renal cell carcinoma (ASPEN). J Clin Oncol 2015;33:abstr 4507.
- Sheng X, Cui C, Chi Z, et al. A pilot, open-label phase II study of sorafenib combined with cisplatin plus gemcitabine for the treatment of patients with advanced renal collecting duct carcinoma. J Clin Oncol 2014;32:abstr e15554.
- Dutcher JP, Nanus D. Long-term survival of patients with sarcomatoid renal cell cancer treated with chemotherapy. Med Oncol 2011;28:1530-3. [PubMed]
- Nanus DM, Garino A, Milowsky MI, et al. Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer 2004;101:1545-51. [PubMed]
- Haas NB, Lin X, Manola J, et al. A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med Oncol 2012;29:761-7. [PubMed]
- Richey SL, Ng C, Lim ZD, et al. Durable remission of metastatic renal cell carcinoma with gemcitabine and capecitabine after failure of targeted therapy. J Clin Oncol 2011;29:e203-5. [PubMed]
- Tannir NM, Thall PF, Ng CS, et al. A phase II trial of gemcitabine plus capecitabine for metastatic renal cell cancer previously treated with immunotherapy and targeted agents. J Urol 2008;180:867-72. [PubMed]
- Ren Z, Zhu K, Kang H, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015;33:894-900. [PubMed]
- He HQ, Xu XJ, Ren ZJ. Percutaneous vertebroplasty for alleviating multiple myeloma–associated pain and preventing paraplegia. Journal of Practical Oncology 2005;20:438-9.
- Diagnosis and Therapy for Bone Metastasis of Malignant Tumors and Skeletal-Related Events Expert Group. Diagnosis and treatment of bone metastasis of renal cancer: an expert consensus statement (2008 version). Zhonghua Zhong Liu Za Zhi 2010;32:317-9. [PubMed]
- Samlowski WE, Majer M, Boucher KM, et al. Multidisciplinary treatment of brain metastases derived from clear cell renal cancer incorporating stereotactic radiosurgery. Cancer 2008;113:2539-48. [PubMed]
- Brinkmann OA, Semik M, Gosherger G, et al. The role of residual tumor resection in patients with metastatic renal cell carcinoma and partial remission following immunotherapy. Eur Urol 2007;6:641-5.
- Patyna S, Peng J. Distribution of sunitinib and its active metabolite in brain and spinal cord tissue following oral or intravenous administration in rodents and monkeys. Eur J Cancer 2006;4:21.
- Minocha M, Khurana V, Qin B, et al. Co-administration strategy to enhance brain accumulation of vandetanib by modulating P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp1/Abcg2) mediated efflux with m-TOR inhibitors. Int J Pharm 2012;434:306-14. [PubMed]
- Henderson CA, Bukowski RM, Stadler WM, et al.The Advanced Renal Cell Carcinoma Sorafenib (ARCCS) expanded access trial: Subset analysis of patients (pts) with brain metastases (BM). J Clin Oncol 2007;25:abstr 15506.
- Gore ME, Hariharan S, Porta C, et al. Sunitinib in metastatic renal cell carcinoma patients with brain metastases. Cancer 2011;117:501-9. [PubMed]
- Donat SM, Diaz M, Bishoff JT. Follow-up for Clinically Localized Renal Neoplasms: AUA Guideline. J Urol 2013;190:407-16. [PubMed]


