Harnessing the innate immune system to treat cancer: enhancement of antibody-dependent cellular cytotoxicity with anti-CD137 Ab
Introduction
Tumor-targeted antibodies are one of the most important developments in the field of cancer therapy in the last 20 years (1). Rituximab was the first monoclonal antibody (mAb) approved in 1997 for the treatment of non-Hodgkin’s lymphoma (2,3). Following rituximab, several mAbs have become standard of care for the treatment of both solid tumors and hematological malignancies, including trastuzumab, alemtuzumab, cetuximab, ofatumumab, pertuzumab (Table 1). Although several effective antibodies have emerged, long-term, durable responses remain elusive and resistance and relapse remain major limitations in cancer therapy (4-6). One of the primary mechanisms of anti-tumor activity of IgG1 monoclonal antibodies is antibody dependent cell-mediated cytotoxicity (ADCC). Many different strategies are under development to stimulate immune effector cells implicated in ADCC in order to enhance the efficacy of tumor-specific mAbs. We have shown that mAbs directed against CD137 enhance natural killer (NK) cell-mediated ADCC against tumor cells. In this review, we discuss some of the promising novel strategies that could potentially enhance ADCC with anti-CD137 mAbs.

Full table
Methods
Efficacy of anti-CD137 mAb was examined by in vitro and in vivo experiments. Peripheral blood mononuclear cells were cultured with SCC6 cells and cetuximab for 24 hours, and flow cytometry analysis was carried out to detect several markers. Clinical trials of anti-CD137 mAb are also on-going.
Results
Antibody dependent cellular cytotoxicity (ADCC)
ADCC plays an important role in the efficacy of IgG1 Abs in cancer therapy (7,8). The specificity of ADCC is conferred by the binding of the antibody through its fragment antigen-binding portion to the tumor-associated antigen on the target cell. Following binding of the mAb to the tumor antigen, the Fc portion of the mAb interacts with the Fc receptor (FcR) on the surface of effector cells (i.e., NK cells and macrophages), and then initiates ADCC. The magnitude of the cytotoxic response is regulated by different classes of activating and inhibiting FcRs. Most hematopoietic cells, except T cells, can express FcγRs (9). FcγRIIIa (CD16) is an activating low-affinity receptor expressed on NK cells and macrophages (10). On the other hand, NK cells do not express the inhibitory FcγRIIb receptors. Without the influence of Fc-mediated inhibitory signalling, NK cells are free to act as key mediators of ADCC in the presence of antibody-coated tumor targets.
Human NK cells comprise around 5% of lymphocytes circulating in the blood and are defined by a CD3−CD56+ phenotype. They are further subdivided into two subsets defined by their expression of CD16: CD56lowCD16+ NK cells and CD56hiCD16− NK cells (11). The CD56lowCD16+ is the predominant subset in the peripheral blood and displays early cytolytic functions, while the CD56hiCD16− cells are distributed in the tissues and secondary lymphoid organs and display a late response, secreting primarily IFN-γ (11).
CD137
CD137 (4-1BB) is a costimulatory receptor that belongs to the TNF receptor superfamily (8,12-14). The cDNA of CD137 was cloned in 1989 as an inducible gene from stimulated T cells (15). Follow-up studies showed that CD137 is also detectable on Tregs, DCs and NK cells. The functional role of CD137 in enhancing cytotoxic T cell responses was established in 1997 (16). Melero et al. (17) first reported that the administration of anti-CD137 mAbs could eradicate established tumors in mice. The immune response induced by anti-CD137 mAbs is mediated by CD8+ cells and accompanied by a marked augmentation of tumor-selective cytolytic T-cell activity. Interestingly, the efficacy of anti-CD137 mAbs was long lasting and generated memory responses as mice survived rechallenge with the same tumor. The role of CD137 in anti-tumor responses was also demonstrated in CD137−/− mice (18). The knockout mice displayed increased metastasis in the lungs and shorter survival time compared with wild type mice. CD137 is a promising therapeutic target for cancer immunotherapy.
Enhancement of ADCC by anti-CD137 Ab
NK cells initiate innate immune responses toward tumor (11,19). Expression of CD137 with several other activation markers (CD69, Tim3) on NK cells increases significantly when NK cells are cultured with mAbs bound to tumor cells (Figure 1) (20-22). We found NK cells in circulation and in the tumors that have upregulated CD137. It was observed that there is a variation between patients in the level of CD137 expression on NK cells after exposure to rituximab in B-cell lymphoma patients (20). Differences in histology and percentage of circulating tumor cells may partially account for this variance, given the observed correlation between circulating tumor cells and CD137 expression levels. In head and neck cancer patients, we also observed a significant increase in NK cell CD137 expression following cetuximab treatment, normally occurring within 24 hours and in some patients, CD137 was elevated as long as 1 week following therapy (22). In addition, patients with either V/V or V/F FcR mutations demonstrated greater CD137 upregulation compared with patients with two low-affinity alleles. We expect anti-CD137 mAb to preferentially target activated NK cells, including NK cells implicated in tumor-directed ADCC, while sparing inactive or resting NK cells, thus limiting the potential off-target toxicity of anti-CD137 mAb.
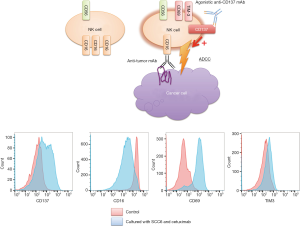
Kohrt et al. (20-22) demonstrated that an anti-CD137 agonistic mAb enhances ADCC and thus the therapeutic activity of anti-tumor mAbs including rituximab, trastuzumab and cetuximab (Figure 1). Human NK cells up-regulate CD137 after encountering mAbs and tumor cells in vitro and in the patients, and subsequent stimulation of these NK cells with anti-CD137 mAb enhances mAb-dependent cytotoxicity against tumor cells (22). Therefore, sequential administration of therapeutic antibodies followed by anti-CD137 mAb with a 24-hour time delay would be better than concurrent administration. Despite the predominant role of NK cells and the innate immune response underlying the mechanism of synergy, we observed a role for CD8+ T cells in the antitumor response. In the combination of anti-CD20 mAb followed by anti-CD137 mAb, increased late relapses were noted when CD8+ T cells were depleted. In vivo, the combination of anti-CD137 mAb and cetuximab increases recruitment of NK cells and dendritic cells to EGFR-expressing tumors, augmenting the innate immune response locally and secondarily enhancing DC function and adaptive immunity with increased tumor infiltration by CD8+ T cells. The systemic nature of the T cell response observed in the mouse model is consistent with findings in the peripheral blood of patients with head and neck carcinoma. Our results further support the tight interplay between the innate and adaptive immune response following mAb therapy. We hypothesize that anti-CD137 agonistic mAb therapy may both stimulate ADCC due to mAb-activated NK cells and promote the proliferation and cytotoxicity of antigen-specific T cells induced by mAb treatment.
Clinical application of anti-CD137 Abs
Two anti-CD137 mAbs have entered clinical testing. Urelumab (BMS-663513) is a fully human IgG4 mAb developed by Bristol-Myers Squibb, and PF-05082566 is a fully human IgG2 mAb developed by Pfizer. They are agonistic mAbs, which bind to the extracellular domain of human CD137.
NCT00309023 study was a first-in-human open-label, ascending, multidose phase I–II trial of urelumab conducted in patients with locally advanced or metastatic solid tumors (23). This study suggested that urelumab was tolerable. Based on the phase I study, a randomized, multi-dose, open-label, phase II study of urelumab as a second-line monotherapy was designed in patients with metastatic melanoma. However, the study was terminated in May 2009 due to fatal hepatotoxicity. Following the first clinical trial, several combination therapies with chemotherapy (NCT00351325), chemoradiation (NCT00461110), ipilimumab (NCT00803374), rituximab (NCT01775631), cetuximab (NCT02110082), and elotuzumab (NCT02252263) have been launched as phase I or I/II studies with a lower dose of urelumab.
Clinical trials of PF-05082566 are also on-going. NCT01307267 is an open-label, dose escalation study that was conducted in patients with advanced malignancies. This study suggested that PF-05082566 was well tolerated, with evidence of disease stabilization in multiple patients.
Other candidates in combination therapy to enhance ADCC
NK cell activation is tightly controlled by combinatorial signalling via a network of activating and inhibitory receptors (7,24). The NKp receptors and leukocyte immunoglobulin-like receptors are solely activating receptors, while the killer cell immunoglobulin-like receptors (KIRs) and CD94-NKG2A receptor family contain both inhibitory and activating receptors (25). The interplay of these activating and inhibiting receptors regulates the responses of NK cells when they encounter potential target cells.
KIR signalling
The KIR family constitutes one of the key mediators of NK cell activation. Inhibitory KIR molecules bind to the self-major histocompatibility complex (MHC) class I ligands (HLA-A, HLA-B, HLA-C) and upon binding transduce inhibitory signals that abrogate the effects of activating receptors (26,27). Because MHC class I is expressed on virtually all healthy cells, KIR molecules are considered to be one of the primary mechanisms responsible for NK cell tolerance to self. Reducing KIR-mediated inhibitory signalling in NK cells via antibody blockade has been shown to increase NK cell cytotoxicity and survival of leukemia-bearing mice. In addition, we reported that blockade of the interface of inhibitory KIRs with MHC class I antigens on lymphoma cells by anti-KIR antibodies prevents a tolerogenic interaction and augments NK-cell spontaneous cytotoxicity (28). Lirilumab, a KIR-blocking mAb (IPH2102/BMS-986015) is currently being tested in clinical trials. Early-phase clinical trials of lirilumab in patients with multiple myeloma demonstrated increased patient-derived NK cell cytotoxicity ex vivo but failed to produce any objective responses (29,30). A trial of lirilumab in patients with acute myeloid leukemia (AML) in first complete remission further validated anti-KIR therapy as safe and tolerable, but only produced transient NK activation (31).
GITR
Glucocorticoid-induced tumor necrosis factor receptor (TNFR) family related gene (GITR) is another member of the TNFR superfamily (TNFRSF18) that is upregulated upon cellular activation (32). GITR ligand (GITRL) is frequently expressed on leukemia cells in AML and chronic lymphocytic leukemia, and impairs the reactivity of NK cells that express GITR (33). GITRL also inhibits the rituximab-induced ADCC of NK cells (34). The anti-GITR mAb TRX518 blocks the interaction of GITR, expressed on NK cells, and its ligand GITRL, thereby increasing the cytotoxicity of NK cells. TRX518 is a promising candidate for combination with other mAbs where it can augment NK cell-mediated ADCC. A phase I study with TRX518 (NCT01239134) is being conducted in melanoma patients.
CD27
CD27, or TNFRSF7, is a costimulatory receptor that is expressed on the surface of T cells, B cells, and NKs, providing a target for enhancing antitumor immunity (35). CD27 signaling is mediated by its cognate ligand, CD70, and CD27-CD70 interactions have been shown to accelerate NK-mediated tumor clearance while generating an adaptive immune response (36). Anti-CD27 therapy dramatically increased ADCC; suggesting innate effectors are integral to the mechanism of 1F5 activity. A phase I trial in patients with solid tumors and hematologic cancers (NCT02270372) is currently ongoing with the anti-CD27 varilumab from Celldex.
BTK inhibitor
Ibrutinib is an irreversible inhibitor of Bruton’s tyrosine kinase (BTK) with promising activity in CD20+ B-cell malignancies and recent US Food and Drug Administration (FDA) approval in mantle cell lymphoma (37). We found that FcR-stimulated NK cells that have been exposed to rituximab-coated lymphoma cells express moderate levels of BTK (38). Ibrutinib inhibited both rituximab- and trastuzumab-induced NK cell cytokine secretion and cytotoxicity. We demonstrate that the abrogation of both rituximab’s and trastuzumab’s antitumor efficacy is a result of ibrutinib’s inhibition of FcR-stimulated NK cell function, specifically ADCC. Selective BTK inhibitors or alternative ibrutinib dosing schedules (e.g., sequential versus concurrent), may preserve the anti-lymphoma efficacy of both agents.
Conclusions
Immunomodulatory antibodies have revolutionized cancer immunotherapy. Most cancer immunotherapy strategies stimulate the patient’s immune system to overcome immunosuppression induced by tumor cells and generate an anti-tumor immune response. The clinical data and recent FDA approvals of ipilimumab, pembrolizumab, nivolumab and blinatumomab validate mAb-mediated cancer immunotherapy as a valuable therapeutic strategy. Agents that augment the antitumor functions of innate immune cells represent a promising new class of immunotherapeutics. Therapies that enhance the antitumor properties of innate effector cells can be combined with antitumor mAbs. One of the most promising findings is the anticancer efficacy of agonistic anti-CD137 mAb. The strong preclinical successes underscore the importance of CD137 in cancer therapy, especially in combination therapeutic strategies. The clinical trials which validate the synergy between established mAbs and co-stimulatory or disinhibitory therapies must emphasize the collection of high-quality biomarkers and immunologic data to better understand this relationship. We believe anti-CD137 mAbs hold great clinical promise. Their clinical potential should be tested in conjunction with other FDA-approved immunomodulators and antibody therapeutics. It is anticipated that “Combination Cancer Immunotherapy” with CD137 will make significant contributions to the field of cancer immunotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science 2013;341:1192-8. [PubMed]
- Maloney DG, Grillo-López AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol 1997;15:3266-74. [PubMed]
- Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 1997;90:2188-95. [PubMed]
- Brufsky AM. Current Approaches and Emerging Directions in HER2-resistant Breast Cancer. Breast Cancer (Auckl) 2014;8:109-18. [PubMed]
- Van Emburgh BO, Sartore-Bianchi A, Di Nicolantonio F, et al. Acquired resistance to EGFR-targeted therapies in colorectal cancer. Mol Oncol 2014;8:1084-94. [PubMed]
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28:4184-90. [PubMed]
- Rajasekaran N, Chester C, Yonezawa A, et al. Enhancement of antibody-dependent cell mediated cytotoxicity: a new era in cancer treatment. ImmunoTargets and Therapy 2015;4:91-100.
- Houot R, Kohrt H, Levy R. Boosting antibody-dependant cellular cytotoxicity against tumor cells with a CD137 stimulatory antibody. Oncoimmunology 2012;1:957-958. [PubMed]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008;8:34-47. [PubMed]
- Trinchieri G, Valiante N. Receptors for the Fc fragment of IgG on natural killer cells. Nat Immun 1993;12:218-34. [PubMed]
- Caligiuri MA. Human natural killer cells. Blood 2008;112:461-9. [PubMed]
- Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther 2012;11:1062-70. [PubMed]
- Li SY, Liu Y. Immunotherapy of melanoma with the immune costimulatory monoclonal antibodies targeting CD137. Clin Pharmacol 2013;5:47-53. [PubMed]
- Yonezawa A, Dutt S, Chester C, et al. Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin Cancer Res 2015;21:3113-20. [PubMed]
- Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A 1989;86:1963-7. [PubMed]
- Shuford WW, Klussman K, Tritchler DD, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med 1997;186:47-55. [PubMed]
- Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med 1997;3:682-5. [PubMed]
- Ju SA, Lee SC, Kwon TH, et al. Immunity to melanoma mediated by 4-1BB is associated with enhanced activity of tumour-infiltrating lymphocytes. Immunol Cell Biol 2005;83:344-51. [PubMed]
- Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol 2013;4:499. [PubMed]
- Kohrt HE, Houot R, Goldstein MJ, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 2011;117:2423-32. [PubMed]
- Kohrt HE, Houot R, Weiskopf K, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest 2012;122:1066-75. [PubMed]
- Kohrt HE, Colevas AD, Houot R, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest 2014;124:2668-82. [PubMed]
- Sznol M, Hodi FS, Margolin K, et al. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). J Clin Oncol 2008;26:abstr 3007.
- Chester C, Marabelle A, Houot R, et al. Dual antibody therapy to harness the innate anti-tumor immune response to enhance antibody targeting of tumors. Curr Opin Immunol 2015;33:1-8. [PubMed]
- Pegram HJ, Andrews DM, Smyth MJ, et al. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 2011;89:216-24. [PubMed]
- Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol 2002;20:217-51. [PubMed]
- Benson DM Jr, Caligiuri MA. Killer immunoglobulin-like receptors and tumor immunity. Cancer Immunol Res 2014;2:99-104. [PubMed]
- Kohrt HE, Thielens A, Marabelle A, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014;123:678-86. [PubMed]
- Benson DM Jr, Cohen AD, Jagannath S, et al. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clin Cancer Res 2015;21:4055-61. [PubMed]
- Benson DM Jr, Hofmeister CC, Padmanabhan S, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012;120:4324-33. [PubMed]
- Vey N, Bourhis JH, Boissel N, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood 2012;120:4317-23. [PubMed]
- Schaer DA, Murphy JT, Wolchok JD. Modulation of GITR for cancer immunotherapy. Curr Opin Immunol 2012;24:217-24. [PubMed]
- Placke T, Kopp HG, Salih HR. Glucocorticoid-induced TNFR-related (GITR) protein and its ligand in antitumor immunity: functional role and therapeutic modulation. Clin Dev Immunol 2010;2010:239083.
- Baltz KM, Krusch M, Bringmann A, et al. Cancer immunoediting by GITR (glucocorticoid-induced TNF-related protein) ligand in humans: NK cell/tumor cell interactions. FASEB J 2007;21:2442-54. [PubMed]
- Hendriks J, Gravestein LA, Tesselaar K, et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol 2000;1:433-40. [PubMed]
- Kelly JM, Darcy PK, Markby JL, et al. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol 2002;3:83-90. [PubMed]
- Herrera AF, Jacobsen ED. Ibrutinib for the treatment of mantle cell lymphoma. Clin Cancer Res 2014;20:5365-71. [PubMed]
- Kohrt HE, Sagiv-Barfi I, Rafiq S, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood 2014;123:1957-60. [PubMed]


