
Literature review of management of brain metastases from germ cell tumors
Introduction
Germ cell tumors (GCT) (seminomatous germ cell tumors, SGCT; and non-seminomatous germ cell tumors, NSGCT) arise in the testes in most cases and commonly metastasize to the regional lymph nodes, lungs, and other sites. Although cisplatin-containing chemotherapy significantly improved outcomes for patients with metastatic GCT, prognosis remains relatively poor for patients with nonpulmonary visceral metastases including brain metastases (1-3). Brain metastases occur in approximately 1 percent of men with disseminated GCT at the time of diagnosis, and approximately 4 percent develop brain metastases after initial treatment (1,4-6). Most brain metastases are in patients with NSGCT (7).
Patients with GCT are not routinely screened for brain metastases at diagnosis but select patients should undergo brain MRI. Advanced tumor stage and multiple or bulky lung metastases have been identified as risk factors for the development of brain metastases from GCT (4). Loriot et al. (8) showed that involvement of the brain was more common among patients previously treated with high-dose chemotherapy (HDCT) (29%) vs. bleomycin, etoposide and cisplatin (BEP) (12%). Brain metastases more commonly occur in the setting of disseminated disease but can occur as the only site of relapse in poor-prognosis GCT (7-9). A prediction model was developed to identify patients at high risk of having brain metastasis. Characteristics implicating higher risk of presence of brain metastasis included: age ≥40, pre-chemotherapy beta-human chorionic gonadotropin (beta-HCG) ≥5,000, choriocarcinoma predominant histology, presence of pulmonary metastasis, and presence of bone metastasis. The risk of having brain metastasis was substantially higher in patients with pulmonary metastases ≥3 cm compared to <3 cm (10). For GCT, brain magnetic resonance imaging (MRI) is clinically indicated per the National Comprehensive Cancer Network (NCCN) guidelines in the presence of neurological symptoms, beta-HCG levels >5,000 IU/L, non-pulmonary visceral metastases, or extensive lung metastases. Additional indications for NSGCT include choriocarcinoma histology or alpha fetoprotein (AFP) >10,000 ng/mL (11).
Survival outcomes vary depending on timing of brain metastases detection; patients with GCT who present with brain metastases at diagnosis have better prognosis than those who develop them at relapse (12-17). Long-term survival probability for patients that have brain metastases at diagnosis ranges from 30–40%. Patients that develop brain metastases after a less than complete response (CR) to chemotherapy and surgery or who develop both brain and extracranial metastases during or after completion of chemotherapy have a cancer-specific survival of approximately 2–26% (13-18). Isolated cerebral relapse after complete remission has been associated with improved outcomes among those with relapsed disease, although this is a rare group (19). An analysis from the Global Germ Cell Cancer Group (GGCCG) of 523 men with brain metastases from a GCT collected retrospectively at 46 centers demonstrated that adverse prognostic factors include multiple brain metastases and liver or bone metastases (for patients with brain metastases at diagnosis or relapse), primary mediastinal seminoma (for patients with brain metastases at diagnosis), and serum beta-HCG ≥5,000 IU/L or AFP ≥100 ng/mL (for patients with brain metastases at relapse) (7). Patients with multiple brain metastases have worse survival outcomes than patients with a single metastasis (14,15). Choriocarcinoma histology is also associated with worse prognosis (9).
In this review article, we discuss the treatment of brain metastases from GCT, including the role of chemotherapy, surgery, and radiation therapy. We present the following article in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-21-127/rc).
Methods
PubMed was searched using the terms ‘CNS metastases’ or ‘brain metastases’ and ‘germ cell’ from 2011 through August 2021. Review articles and randomized clinical trials related to the treatment of brain metastases in GCT were included. Additional hand searches were done of the article references and clinical practice guidelines for testicular cancer or GCT including the NCCN guidelines, UpToDate, European consensus guidelines, and Canadian consensus guidelines.
Management
No prospective data adequately address the optimal treatment sequence for brain metastases in GCT; thus, management decisions are generally based on institutional experience and expert opinion. GCT patients with brain metastases are a unique and rare population given the young average age at diagnosis and high probability for long term survival. European consensus guidelines and NCCN guidelines recommend immediate upfront chemotherapy at initial diagnosis, but there is not a consensus on additional surgery or radiation (11,20). For patients relapsing with brain metastases, the majority recommend full salvage chemotherapy (20). The NCCN guidelines recommend primary cisplatin-based chemotherapy ± radiation therapy ± surgery (11). Canadian consensus guidelines recommend that patients receive 4 cycles of BEP (21).
Role of chemotherapy—initial treatment
For patients with brain metastases at initial diagnosis, systemic chemotherapy is often administered alone for initial treatment. In selected cases, chemotherapy may be used in combination with radiation therapy and/or surgical resection if clinically indicated, primarily when patients are significantly symptomatic (11). Cisplatin and etoposide penetrate the brain to a reasonable degree; thus, standard-dose systemic chemotherapy [BEP, or etoposide, ifosfamide, and cisplatin (VIP)] is used initially prior to any consideration for resection or radiation therapy (22). If repeat brain imaging following completion of first-line chemotherapy shows a CR, careful observation without further brain-directed treatment is our institutional preference. If there is a limited amount of residual tumor following chemotherapy, surgical excision and/or focal radiation therapy can be used to eradicate residual disease (22).
Role of chemotherapy—intensification
HDCT has been investigated for upfront therapy. Kollmannsberger et al. (9) reported outcomes of patients with GCT brain metastases at initial diagnosis enrolled on a German multicenter trial between January 1993 and July 1998, where all 22 patients received first-line HDCT with VIP (one cycle of standard-dose, followed by 3–4 cycles with dosages escalated over seven dose levels) followed by autologous stem-cell transplantation. Whole brain radiation therapy (WBRT) to 30–50 Gy (±10 Gy boost) was applied in patients with symptomatic disease or as consolidation for residual brain metastases. Two early deaths occurred, one with central nervous system (CNS) bleeding, and one from sepsis. Twenty patients (91%) had a response in the brain [55% CR/36% partial response (PR)]. Two-year progression-free survival (PFS) and overall survival (OS) were 72% and 81%, respectively.
However, a randomized phase 3 trial of 219 patients from the United States comparing conventional BEPx4 vs. 2 cycles of BEP followed by 2 cycles of HDCT (carboplatin 600 mg/m2, etoposide 600 mg/m2, and cyclophosphamide 50 mg/kg) and subsequent stem cell rescue as first-line treatment for poor prognosis GCT did not show an improvement in the primary endpoint of 1-year durable CR (52% CR after BEP + HDCT vs. 48% CR with BEP alone, P=0.53). For the subset of 67 patients with unsatisfactory serum tumor marker decline (half-life >7 days for AFP and/or half-life >3.5 days for beta-HCG) during the first two cycles of chemotherapy, the 1-year durable CR was 61% for patients who received HDCT vs. 34% for patients with BEP alone (P=0.03), suggesting a benefit for this population (23).
A phase III study of 137 patients from 27 European oncology centers that randomized patients with poor-prognosis GCT to BEPx4 vs. HDCT with one cycle of standard dose VIP and then high-dose VIPx3 (etoposide 1,500 mg/m2, ifosfamide 12 g/m2, and standard-dose cisplatin) followed by stem cell infusion was also negative for the primary endpoint of failure-free survival (FFS). The intention to treat analysis included 131 patients and found hazard ratio (HR) for FFS of 0.62, in favor of HDCT [95% confidence interval (CI): 0.38–1.02; P=0.057]. The lack of statistical significance was noted to likely be due to premature study termination for slow accrual. The 1-year FFS rate was 48% (95% CI: 35.5–59.5%) after BEP and 66.1% (95% CI: 53.1–76.2%) after HDCT, P=0.035. FFS rate at 2 years and OS did not demonstrate a statistically significant difference. In contrast to the US intergroup study, there was a suggestion for a greater benefit from HDCT in patients with satisfactory marker decline in a post hoc subgroup analysis. Toxicity was increased with HDCT, and of the three patients that died from toxicity, two patients were on the HDCT arm (24).
GETUG-13 was a phase III randomized trial primarily conducted in France where poor-prognosis GCT patients received one cycle of BEP after which AFP and beta-HCG levels were assessed and then patients with a favorable decline in both tumor markers (based on a logarithmic formula) received BEPx3, while patients with an unfavorable decline were randomized to either BEPx3 or a dose-dense regimen. Dose-dense chemotherapy is another way to escalate therapy by shortening the interval between the doses. This regimen consisted of paclitaxel (175 mg/m2) on day 1 prior to BEPx3 plus oxaliplatin (130 mg/ m2 on day 10 for 2 cycles), followed by 2 cycles of cisplatin (100 mg/m2), ifosfamide (2 g/m2), and bleomycin (25 units/day by continuous infusion for 5 days) with granulocyte colony-stimulating factor (G-CSF) support. The study enrolled 263 patients, among which 203 were randomized due to unfavorable tumor marker decline (105 to dose-dense arm, and 98 to standard BEP arm) and found 3-year PFS of 59% in the dose-dense arm vs. 48% in the standard BEP arm [P=0.05; HR =0.66 (95% CI: 0.44–1.00)]. PFS was significantly improved for patients with a favorable decline in tumor markers that were not randomized and received BEPx3 compared to those with an unfavorable decline that were randomized to BEP with 3-year PFS of 70% in the favorable arm (95% CI: 57–81%). OS difference was not statistically significant. Higher rates of toxicity occurred in the dose-dense arm, with more hematologic effects and grade 3–4 neurotoxicity, but no excess febrile neutropenia or deaths due to toxicity. A post hoc analysis suggested isolated brain progression may be more common in patients treated with dose-dense chemotherapy compared to standard BEP (25).
For patients with IGCCG poor risk disease including patients with brain metastasis, first-line BEPx4 or VIPx4 remains standard, but treatment intensification may be considered on an individual basis. Per the German GCT guidelines, HDCT is not generally recommended for all poor-prognosis patients, but those with mediastinal NSGCT should undergo HDCT and HDCT should be considered on an individual basis for other GCT patients that have an insufficient tumor marker decline after 1 or 2 cycles of cisplatin-based first-line therapy (grade B, moderate quality of evidence) (26). GCT are vascular (especially choriocarcinoma) and thus treatment with chemotherapy (especially with HDCT) in the setting of brain metastases requires very close monitoring of platelets and monitoring for neurologic symptoms (22).
Role of chemotherapy—relapse
For patients with brain metastases at relapse after first-line chemotherapy, standard treatment involves salvage HDCT or standard-dose chemotherapy followed by consideration for resection of residual masses. If residual masses are not amenable to resection, then these patients should be evaluated for either stereotactic radiosurgery (SRS) or WBRT (27). The GGCCG reported that multimodality treatment was associated with improved survival in comparison with a single modality for patients with brain metastases at relapse (HR =0.51; P<0.001), as was high-dose compared with standard-dose chemotherapy (HR =0.41; 95% CI: 0.24–0.70; P=0.001) (7). A retrospective analysis by Kalra et al. (28) reported outcomes of 25 patients with relapsed GCT and progressive brain metastases who received multimodality therapy at Indiana University from 2006 to 2016 including HDCT with stem cell support alone or combined with surgery and/or SRS or WBRT. HDCT consisted of carboplatin 700 mg/m2 on days 1 to 3 with etoposide 750 mg/m2 on days 1 to 3, followed by peripheral blood stem cell transplantation on day 5 for 2 cycles. Three patients received only 1 course of HDCT, and two patients died of complications during therapy from fungemia and septic shock. Most patients did not undergo radiation after HDCT except for 2 patients that underwent consolidative WBRT and 2 that underwent SRS. At median follow-up of 24.5 months, 44% of patients were alive without evidence of disease, demonstrating cure of a subset of patients with historically poor prognosis (28). A study by Oechsle et al. (29) reported that an interdisciplinary approach of HDCT, brain radiation (in 90% of patients) and surgery led to long-term survival in 60% of patients with brain metastases at diagnosis and 26% relapsing with brain metastases.
Role of chemotherapy—oligometastatic disease
Some patients relapse with a solitary brain metastasis with no other systemic disease. In these situations, a multidisciplinary approach is important, and our institutional preference is resection of solitary brain metastasis if feasible. Given relapse with brain metastasis usually heralds a systemic relapse, we usually recommend systemic adjuvant chemotherapy after local therapy to the brain.
Role of surgery
There is considerable debate about the role of surgery and prospective trials are lacking. Due to the rarity of GCT brain metastases, it is very challenging to provide strong recommendations. Surgical excision is generally reserved for the setting of limited amount of residual tumor following chemotherapy or in the setting of relapse for men with a solitary brain metastasis as the only site of relapse. A solitary brain metastasis relapse can alternatively be treated by further chemotherapy followed by either resection or SRS.
In NSGCT, post-chemotherapy residual disease may be recommended for resection depending on factors such as location and size of residual disease, whether residual disease is symptomatic, whether tumor markers normalized after chemotherapy, and others. Our institutional preference is surveillance for patients who achieve a post-chemotherapy residual mass <1 cm based on long-term data confirming the safety of this approach, acknowledging most of the data is on residual retroperitoneal disease, with few brain metastases patients included (30,31).
Surgical resection is advocated for cases of large metastases (>3 cm)and those causing significant mass effect or brain shift (14). There are proponents for surgical resection of brain metastases with significant elements of choriocarcinoma that may have been defined by pathology at another body site or high levels of serum beta-HCG (32). This recommendation may be warranted as there is an increased tendency for choriocarcinoma to spontaneously bleed, and surgical resection can help avoid a potential life-threatening intracranial hemorrhage. Fosså et al. (5) reported that use of neurosurgery was correlated significantly with improved prognosis for patients with brain metastases at diagnosis, with a 2-year survival of 80% for patients that underwent neurosurgery (n=10) vs. 49% for patients that did not (n=46), P=0.021. For patients that underwent neurosurgical resection for brain metastases that developed after cisplatin-based chemotherapy, neurosurgical resection was significantly related to improved 2-year survival (P<0.001) but was not significant on multivariate analysis (5).
Role of radiation
Radiation may be indicated for patients with numerous brain metastases that do not resolve with chemotherapy or highly symptomatic patients who are not candidates for resection. No prospective studies have investigated the use of radiation. Fosså et al. (5) demonstrated no improvement in prognosis with use of brain radiation for brain metastases present at diagnosis in a retrospective analysis. However, the study did show an improvement in prognosis for brain metastases that developed after patients underwent cisplatin-based chemotherapy—59 patients underwent radiation with 2-year survival of 18% and 24 did not undergo radiation with 2-year survival of 8%, P=0.042. Feldman et al. (7) retrospectively looked at 523 men with GCT brain metastases (228 patients with brain metastases at diagnosis and 295 patients with brain metastases at the time of relapse); 55% of patients in the brain metastasis at diagnosis group received multimodality treatment including neurosurgical resection and/or radiation in addition to chemotherapy. This approach showed an improved outcome compared to single modality treatment, but this benefit was lost when adjusted for the prognostic groups defined in the paper based on a scoring system incorporating adverse prognostic factors. However, WBRT was not associated with significant improvement in OS. In patients with relapsed brain metastases, all treatment modalities including chemotherapy alone, HDCT alone, surgery alone, and radiation therapy alone were associated with improved OS in univariate analysis, but only multimodality treatment and HDCT improved OS in multivariate analysis. Subset analyses stratified by prognostic groups showed the intermediate risk group benefited the most from multimodal treatment.
Concurrent WBRT and cisplatin-based chemotherapy has also been tried in the past. A study by Bokemeyer et al. (33) demonstrated that patients who received combined chemotherapy and radiation with or without surgery had the highest chance of long-term survival, P<0.03. However, this finding is likely biased by the fact that patients with worse performance status or disease status were more likely to receive single modality treatment. A report from Indiana University described 24 GCT treated with this approach; however, the sample size was small and only 6 of 24 patients were alive and at risk of delayed toxicity at time of publication (13). An updated report revealed that 5 patients who underwent WBRT to 40–50 Gy in 18–28 fractions concurrent with cisplatin all developed delayed symptoms including seizures, cranial neuropathy, headaches, and dementia with imaging changes consistent with a diagnosis of progressive multifocal leukoencephalopathy. One patient developed a secondary malignant glioma within an area of radiation necrosis (17).
Biologically effective dose (BED) is another factor that is important in terms of the success of radiation treatment. BED is a measure of the true biological dose delivered by a combination of the dose per fraction and total dose to a tissue, with each tissue having a characteristic α/β ratio. The significance of BED was shown by Casey et al. (34) in a retrospective review of patients at MSKCC from 2002–2017 including 63 patients with NSGCT and brain metastases (15 patients with tumor at diagnosis and the rest developing metastases later). The radiation technique used in this study was WBRT (71%), SRS (11%), WBRT + SRS/boost (14%), and partial brain radiation (1%). The 4-year OS rate was 30.1%. This study showed an association between OS and BED (with α/β ratio of 10). BED less than 39 Gy (equivalent to 30 Gy in 10 fractions, the typical dose used for WBRT) was associated with 0% 4-year OS and the OS increased to 66.7% when BED was more than 50 Gy. The lack of response to WBRT with a BED of 39 Gy is likely due to NSGCT being more radioresistant in comparison to other histologies such as SGCT. Unlike other diseases like lung cancer in which patients typically receive WBRT with a palliative intent, patients with NSGCT potentially can be cured, emphasizing the need to use a radiation dose that both achieves durable disease control and minimizes long term toxicity. Another important factor in this study that correlated with long-term survival was intracranial control after finishing radiation (34).
As mentioned in the above studies, SRS is an option for treating these patients and Gamma Knife (GK) can be used in this setting. In a study by Nicolato et al. (35), GK was used to treat patients with NSGCT from 1995–2001. This case series included three patients who received cisplatin-based chemotherapy and WBRT followed by GK. Indications for GK were tumor volume <20 cm3, refusal of surgery and microsurgery too risky. The mean dose was 17.3 Gy with a range of 15–21 Gy. After a median follow-up of 63 months, all patients were alive. In this study, an algorithm was proposed. If patients had ≤4 lesions with <20 cc overall volume SRS was recommended. If there were >4 lesions and >20 cc, WBRT with and without microsurgery was suggested (35).
Given that GCT patients are young with a potentially curable disease even in the presence of brain metastasis, the late effects of radiation in addition to chemotherapy ± surgery are important to consider. When radiation is indicated, our strong institutional preference is to avoid WBRT and treat with localized radiation. However, surveillance is a strong consideration as well. A study by Gremmer et al. (16) evaluated results of treatment of patients with GCT brain metastases over a period at a single institution that initially included but later excluded WBRT. Patients were divided into three groups; group 1 (n=10) consisted of patients with brain metastases at diagnosis, with CR achieved in five of ten patients with follow-up of 49–245 months (one of five patients that underwent WBRT, and four of five patients without WBRT with two of these receiving additional HDCT). Group 2 (n=2) were patients who relapsed with brain metastases after CR following induction chemotherapy, both patients underwent neurosurgical resection (one patient received additional HDCT with WBRT, and the other HDCT without WBRT) and both patients had CR and remained without disease at follow-up of 146 and 211 months. Group 3 (n=10) were patients who developed brain metastases after failing to achieve a CR at induction chemotherapy, one patient underwent neurosurgical resection, seven patients received WBRT, and four patients had additional HDCT, and one of ten patients achieved CR after HDCT and WBRT (follow-up 107 months) (16). Another study by Hardt et al. (36) assessed the outcome in GCT patients with brain metastases after the introduction of GAMEC chemotherapy (14-day cisplatin, high-dose methotrexate, etoposide, and actinomycin-D) and cessation of the routine use of brain radiation, which showed a 3-year OS rates of 69% for those who presented with brain metastases at diagnosis, 21% for those who developed metastases after initial chemotherapy, and 0% for those who developed metastases while receiving chemotherapy. These results demonstrate that long-term cure is possible without radiation.
WBRT may still be utilized in a palliative setting. For example, in patients with neurologic symptoms such as headache, nausea, vomiting, or focal neurologic deficit, especially in the setting of extensive brain disease in a poor surgical candidate, palliative WBRT may result in stable or improved neurologic symptoms in a subset of patients (37). Best supportive care including dexamethasone is another option (38).
Conclusions
Since brain metastasis from GCT are rare, there are no consensus recommendations to guide physicians. In this review, all available articles were discussed to help physicians make appropriate therapeutic decisions tailored to each patient’s clinical situation. MRI of the brain is important to consider at GCT diagnosis for patients with neurological symptoms and/or high-risk features including beta-HCG levels >5,000 IU/L, non-pulmonary visceral metastases, extensive lung metastases, choriocarcinoma histology, or AFP >10,000 ng/mL. After appropriate diagnostic work-up and staging, multi-agent chemotherapy is the first-line therapy for these patients with brain metastases at diagnosis. Intensification of chemotherapy with high-dose or dose-dense regimens is non-standard but can be considered in specific cases, such as insufficient tumor marker decline during standard chemotherapy. Further research may clarify subsets of patients that would benefit most from more intense regimens. Patients who present with symptomatic disease and need urgent CNS-directed therapy should be considered for CNS-directed therapy, with chemotherapy to be initiated shortly thereafter.
In the setting of residual brain metastasis after chemotherapy, multidisciplinary discussion with neurosurgery and radiation oncology is indicated to determine appropriate adjuvant/salvage treatment. Based on the study by Feldman et al. (7), multimodality treatment can improve outcomes. Therefore, surgical resection with or without radiation treatment should be discussed with patients. It is important to remember that a higher BED can improve OS and thus SRS should be considered on an individual basis (34).
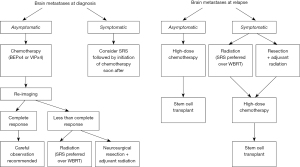
In patients with metachronous brain metastases, it is important to evaluate extracranial disease burden. If the patient has metastases in the brain only, treatment options include (I) surgical resection ± radiation; or (II) SRS alone. SRS can be used in 1–4 lesions or when the cumulative volume is less than 20 cc. SRS can also be considered in more than four lesions on a case-by-case basis. If not, WBRT is an option for these patients. Our recommendations are summarized in Figure 1.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Chinese Clinical Oncology, for the series “The Modern Approaches to the Management of Brain Metastases”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-21-127/rc
Conflicts of Interest: All authors except for GAW have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-21-127/coif). The series “The Modern Approaches to the Management of Brain Metastases” was commissioned by the editorial office without any funding or sponsorship. KS served as the unpaid Guest Editor of the series. We are unable to provide a conflict of interest statement for GAW. Our beloved friend and colleague GAW unfortunately passed away during the editorial phase of this submission and will be dearly missed. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997;15:594-603. [Crossref] [PubMed]
- Adra N, Althouse SK, Liu H, et al. Prognostic factors in patients with poor-risk germ-cell tumors: a retrospective analysis of the Indiana University experience from 1990 to 2014. Ann Oncol 2016;27:875-9. [Crossref] [PubMed]
- Gillessen S, Sauvé N, Collette L, et al. Predicting Outcomes in Men With Metastatic Nonseminomatous Germ Cell Tumors (NSGCT): Results From the IGCCCG Update Consortium. J Clin Oncol 2021;39:1563-74. [Crossref] [PubMed]
- Raina V, Singh SP, Kamble N, et al. Brain metastasis as the site of relapse in germ cell tumor of testis. Cancer 1993;72:2182-5. [Crossref] [PubMed]
- Fosså SD, Bokemeyer C, Gerl A, et al. Treatment outcome of patients with brain metastases from malignant germ cell tumors. Cancer 1999;85:988-97. [Crossref] [PubMed]
- Raghavan D, Mackintosh JF, Fox RM, et al. Improved survival after brain metastases in non-seminomatous germ cell tumours with combined modality treatment. Br J Urol 1987;60:364-7. [Crossref] [PubMed]
- Feldman DR, Lorch A, Kramar A, et al. Brain Metastases in Patients With Germ Cell Tumors: Prognostic Factors and Treatment Options--An Analysis From the Global Germ Cell Cancer Group. J Clin Oncol 2016;34:345-51. [Crossref] [PubMed]
- Loriot Y, Pagliaro L, Fléchon A, et al. Patterns of relapse in poor-prognosis germ-cell tumours in the GETUG 13 trial: Implications for assessment of brain metastases. Eur J Cancer 2017;87:140-6. [Crossref] [PubMed]
- Kollmannsberger C, Nichols C, Bamberg M, et al. First-line high-dose chemotherapy +/- radiation therapy in patients with metastatic germ-cell cancer and brain metastases. Ann Oncol 2000;11:553-9. [Crossref] [PubMed]
- Ashkar R, Althouse SK, Cary C, et al. Prediction model for brain metastasis (BM) in patients with metastatic germ-cell tumors (mGCT) accounting for size of pulmonary metastases. J Clin Oncol 2021;39:378. [Crossref]
- Testicular Cancer: National Comprehensive Cancer Network 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/testicular.pdf
- Boyle HJ, Jouanneau E, Droz JP, et al. Management of brain metastases from germ cell tumors: a single center experience. Oncology 2013;85:21-6. [Crossref] [PubMed]
- Spears WT, Morphis JG 2nd, Lester SG, et al. Brain metastases and testicular tumors: long-term survival. Int J Radiat Oncol Biol Phys 1992;22:17-22. [Crossref] [PubMed]
- Salvati M, Piccirilli M, Raco A, et al. Brain metastasis from non-seminomatous germ cell tumors of the testis: indications for aggressive treatment. Neurosurg Rev 2006;29:130-7. [Crossref] [PubMed]
- Nonomura N, Nagahara A, Oka D, et al. Brain metastases from testicular germ cell tumors: a retrospective analysis. Int J Urol 2009;16:887-93. [Crossref] [PubMed]
- Gremmer R, Schröder ML, Ten Huinink WW, et al. Successful management of brain metastasis from malignant germ cell tumours with standard induction chemotherapy. J Neurooncol 2008;90:335-9. [Crossref] [PubMed]
- Doyle DM, Einhorn LH. Delayed effects of whole brain radiotherapy in germ cell tumor patients with central nervous system metastases. Int J Radiat Oncol Biol Phys 2008;70:1361-4. [Crossref] [PubMed]
- Schmoll HJ, Souchon R, Krege S, et al. European consensus on diagnosis and treatment of germ cell cancer: a report of the European Germ Cell Cancer Consensus Group (EGCCCG). Ann Oncol 2004;15:1377-99. [Crossref] [PubMed]
- Lutterbach J, Spetzger U, Bartelt S, et al. Malignant germ cell tumors metastatic to the brain: a model for a curable neoplasm? The Freiburg experience and a review of the literature. J Neurooncol 2002;58:147-56. [Crossref] [PubMed]
- Beyer J, Albers P, Altena R, et al. Maintaining success, reducing treatment burden, focusing on survivorship: highlights from the third European consensus conference on diagnosis and treatment of germ-cell cancer. Ann Oncol 2013;24:878-88. [Crossref] [PubMed]
- Wood L, Kollmannsberger C, Jewett M, et al. Canadian consensus guidelines for the management of testicular germ cell cancer. Can Urol Assoc J 2010;4:e19-38. [Crossref] [PubMed]
- Gilligan TD. Diagnosis and treatment of relapsed and refractory testicular germ cell tumors. UpToDate 2021. Available online: https://www.uptodate.com/contents/diagnosis-and-treatment-of-relapsed-and-refractory-testicular-germ-cell-tumors?source=history_widget.#H19
- Motzer RJ, Nichols CJ, Margolin KA, et al. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol 2007;25:247-56. [Crossref] [PubMed]
- Daugaard G, Skoneczna I, Aass N, et al. A randomized phase III study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974). Ann Oncol 2011;22:1054-61. [Crossref] [PubMed]
- Fizazi K, Pagliaro L, Laplanche A, et al. Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial. Lancet Oncol 2014;15:1442-50. [Crossref] [PubMed]
- Kliesch S, Schmidt S, Wilborn D, et al. Management of Germ Cell Tumours of the Testes in Adult Patients: German Clinical Practice Guideline, PART II - Recommendations for the Treatment of Advanced, Recurrent, and Refractory Disease and Extragonadal and Sex Cord/Stromal Tumours and for the Management of Follow-Up, Toxicity, Quality of Life, Palliative Care, and Supportive Therapy. Urol Int 2021;105:181-91. [Crossref] [PubMed]
- Gilligan T. Decision Making in a Data-Poor Environment: Management of Brain Metastases From Testicular and Extragonadal Germ Cell Tumors. J Clin Oncol 2016;34:303-6. [Crossref] [PubMed]
- Kalra M, Adra N, Hanna N, et al. High-dose chemotherapy plus peripheral blood stem cell transplantation for patients with relapsed germ cell tumors and active brain metastases. Cancer 2020;126:1202-7. [Crossref] [PubMed]
- Oechsle K, Kollmannsberger C, Honecker F, et al. Cerebral metastases in non-seminomatous germ cell tumour patients undergoing primary high-dose chemotherapy. Eur J Cancer 2008;44:1663-9. [Crossref] [PubMed]
- King J, Althouse SK, Cary C, et al. Surveillance after complete response in patients with metastatic non-seminomatous germ-cell tumor (NSGCT). J Clin Oncol 2021;39:5018. [Crossref]
- Kollmannsberger C, Daneshmand S, So A, et al. Management of disseminated nonseminomatous germ cell tumors with risk-based chemotherapy followed by response-guided postchemotherapy surgery. J Clin Oncol 2010;28:537-42. [Crossref] [PubMed]
- Riggs SB, Burgess EF, Gaston KE, et al. Postchemotherapy surgery for germ cell tumors--what have we learned in 35 years? Oncologist 2014;19:498-506. [Crossref] [PubMed]
- Bokemeyer C, Nowak P, Haupt A, et al. Treatment of brain metastases in patients with testicular cancer. J Clin Oncol 1997;15:1449-54. [Crossref] [PubMed]
- Casey DL, Pitter KL, Imber BS, et al. High-dose radiation therapy is needed for intracranial control and long-term survival in patients with non-seminomatous germ cell tumor brain metastases. J Neurooncol 2019;142:523-8. [Crossref] [PubMed]
- Nicolato A, Ria A, Foroni R, et al. Gamma knife radiosurgery in brain metastases from testicular tumors. Med Oncol 2005;22:45-56. [Crossref] [PubMed]
- Hardt A, Krell J, Wilson PD, et al. Brain metastases associated with germ cell tumors may be treated with chemotherapy alone. Cancer 2014;120:1639-46. [Crossref] [PubMed]
- Bezjak A, Adam J, Barton R, et al. Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer 2002;38:487-96. [Crossref] [PubMed]
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004-14. [Crossref] [PubMed]


