Korean perspectives of nasopharynx cancer management
Introduction and epidemiology
Nasopharynx cancer (NPCa) is usually distinguishable from other malignancies arising in the head and neck region because of its uniqueness with respects to epidemiology, histologic type, clinical behavior, and treatment. According to the global cancer statistics, the estimated crude incidence of NPCa in the year of 2008 was 84,400, which represented about 0.7% of all cancer burdens (1). It was reported that there existed great variations in the age-adjusted incidence rates depending on the geography and the ethnicity. The highest incidence rate was reported in South-Eastern Asia (6.5 and 2.8 per 100,000 male and female) and the lowest was in Central America (0.2 and 0.1 per 100,000 male and female). According to the Korean national cancer statistics in the year of 2013 (2), the crude incidence of all types of cancers was 225,343 and that of NPCa was 416. As a result of unnecessary screening and overdiagnosis, the incidence of thyroid cancer was very high [42,541] enough to deviate the vital statistics in Korea (3). After excluding the incidence of thyroid cancer, the NPCa incidence accounted for 0.2% of all cancer types and 9.1% of all malignancies arising in the head and neck regions. Though Korea is located in Eastern Asia and very close China, the incidence rate was 0.6 per 100,000 general Korean people, which was, more or less, higher than in Western countries, and, however, significantly lower than in South-Eastern Asia.
Epstein-Barr virus (EBV) infection prevalence and histologic subtype
Histologic subtypes of NPCa are known to vary depending on the geographic regions, which closely correlates with the prevalence of EBV infection. Non-keratinizing subtype is most common in EBV endemic regions including Southern China, Hong Kong, and Singapore, and, on the other hand, keratinizing subtype is more frequent in North America (4). Though Korea belongs to the non-endemic region of EBV infection and the incidence rate is remarkably low, some clinicopathologic characteristics, however, seem to be shared with those in the endemic region in that there is strong correlation with EBV infection and that more frequent non-keratinizing subtype (5,6).
Establishment of Korean database
Though there have been occasional changes in the treatment guidelines of NPCa, high dose radiation therapy (RT) has long been the main treatment modality in NPCa: RT alone for the patients at stage I and concurrent chemoradiotherapy (CCRT) for those at higher stages (7). Though the updated guidelines have been easily available through the internet, it cannot be denied that the actual therapeutic strategies have not been standardized throughout Korea. Considering that the annual number of newly diagnosed NPCa patients has been around 400 while the number of RT facilities recently have become over 80, the NPCa patients receiving RT has never been evenly distributed to the RT facilities within Korea. In order to see how the NPCa patients had been managed in Korea, Korean Radiation Oncology Group (KROG) decided to establish a retrospective database. Fifteen radiation oncology departments participated in this project and the clinical and treatment data of 1,476 patients, who were given high dose RT from September 1988 till October 2011, were collected.
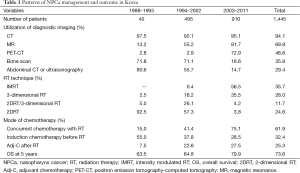
Patterns of care study
Data of 1,445 patients were used to assess the patterns of care and the clinical outcome analyses (8). Following staging work-up’s, the numbers of patients assigned to AJCC stages I, II, III, and IV were 71 patients (4.9%), 279 (19.3%), 577 (39.9%), and 518 (35.8%), respectively. The time frame was arbitrarily divided into 3 periods: the first period was from 1988 till 1993, when 2-dimensional RT (2DRT) technique was popular but 3-dimensional conformal RT (3DCRT) technique was not; the second period was from 1994 till 2002, when 3DCRT was popularized but intensity modulated RT (IMRT) technique was not; and third period was from 2003 till 2011, when IMRT became popularized (Table 1). During the first period, 2DRT was applied to the vast majority (97.5%) of the patients (as a sole RT modality in 92.5% and as a part of RT course in 5.0%). During the third period, on the contrary, the up-to-date techniques of 3DCRT and IMRT were utilized to the vast majority (92.0%) of the patients (35.5% and 56.5%, respectively), while 2DRT was utilized to only minority (8.0%) of patients (as a sole RT modality in 3.8% and as a part of RT course in 4.2%), respectively. As the imaging tool for local and regional disease extent verification, utilization of magnetic resonance (MR), in addition to CT, gradually increased over time, meanwhile, bone scan and abdominal CT or ultrasonography for systemic staging dramatically decreased and were largely replaced by positron emission tomography-computed tomography (PET-CT) during the third period. Systemic chemotherapy was added to RT typically in those at advanced stages, and the modes of chemotherapy in relation to RT changed over the time frame: concurrent administration of chemotherapy during RT (CCRT) has become more frequent and popularized (15.0%, 41.4%, and 75.1%); while neoadjuvant chemotherapy (Neo-C) has become less and less frequent (55.0%, 37.8%, and 28.5%). The overall survival (OS) rate at 5 years during the third period was significantly improved when compared to those during the first and second periods (63.5% vs. 64.8% vs. 79.9%, P<0.001). This improvement in survival seemed to have been the reflection of improvements in diagnostic imaging (MR and PET-CT in addition to CT), RT technique (IMRT), and mode of systemic chemotherapy administration (CCRT).
Full table
Treatment patterns and clinical outcomes in cN0 patients
The proportion of NPCa patients diagnosed without lymph node metastasis (cN0) is generally very low. As there have not been enough reports in this clinical setting, no consensus opinion on the optimal treatment strategy and the role of systemic chemotherapy has been available. Among 1,045 patients who were registered to the database from 2000 till 2011, 148 (14.2%) received upfront RT for having cN0 disease (9). cT stages were cT1 in 54 patients (36.5%), cT2 in 29 (19.6%), cT3 in 24 (16.2%), and cT4 in 41 (27.7%), respectively, and treatment schemes were RT alone in 70 patients (47.3%) and CCRT in 78 (52.7%), respectively. Adjuvant chemotherapy (Adj-C) was added to 29 patients (19.6%), all of who received CCRT for having cT2-4. Treatment patterns differed significantly depending on cT stages. RT alone was more commonly applied to the patients with cT1 (43/54, 79.6%), while CCRT was to those with cT2-4 (67/94, 71.3%), respectively. The 5-year rates of OS, locoregional (LR) control, disease-free (DF) survival all cN0 patients were 75.4%, 79.9%, 87.6%, and 69.5%, respectively (Table 2). In multivariate analyses, cT1 patients achieved significantly better OS (100% vs. 62.3%, P=0.006) and DF survival (88.7% vs. 57.9%, P<0.001) than those with cT2-4 regardless of chemotherapy and CCRT was significantly advantageous over RT alone with respects to LR control (77.0% vs. 83.0%, P=0.008) and DF survival (66.4% vs. 71.8%, P=0.029). Adj-C applied following RT or CCRT to cT2-4 patients, however, failed to improve any of the clinical outcomes. Consequently, RT alone seemed sufficient to the patients with cT1 and CCRT alone (without Adj-C) is highly recommended to those with cT2-4.

Full table
Role of chemotherapy in stage II
From the database, 138 patients were selected, who received high RT with or without chemotherapy at 12 departments from 2004 till 2011 for having stage II (cT1N1 or cT2N0-1) (10). Treatment methods included RT alone in 34 patients (24.6%), Neo-C + RT in seven (5.1%), CCRT in 80 (58.0%), and Neo-C + CCRT in 17 (12.3%), respectively. Adj-C was optionally added following RT or CCRT in 42 patients (30.4%). The 5-year rates of OS, LR control, DF survival of all patients were 88.2%, 86.2%, and 74.4%, respectively. Multivariate analyses showed that CCRT significantly improved LR control (P<0.001) and DF survival (P=0.012). Neo-C failed to improve any of the clinical outcomes and concurrent chemotherapy failed to reduce distant metastasis or to increase OS. CCRT alone seemed to be optimal in treating stage II patients.
Role of neoadjuvant and Adj-C in locoregionally advanced stages
Neo-C has the rationales of reducing tumor burden before initiating definitive local treatment and eradicating probable micro-metastases at the same time. Several phase II trials reported high overall response rate following cisplatin-based combination chemotherapy ranging from 75–90% (11-13), however, prospective randomized trials failed to demonstrate survival benefit (14-16). Influenced by Intergroup study 0099 (17), Adj-C was frequently employed in several clinical trials investigating the efficacy of CCRT (18,19). Survival benefit, however, was not demonstrated by Adj-C either. Meta-analysis reported in 2015 by Yan et al. (20) showed that there was survival benefit neither by Neo-C nor by Adj-C when compared with CCRT alone.
Among total 1,445 patients registered to the database, systemic chemotherapy was concurrently delivered during the RT course (CCRT) in 895 patients (61.9%) as the main treatment for having locoregionally advanced stages. Among these, CCRT alone was given in 380 patients (42.5%), Neo-C was given before CCRT in 188 (21.0%), and Adj-C was added following CCRT in 327 (36.5%), respectively (Figure 1). The roles of Neo-C and Adj-C in conjunction with CCRT were assessed through 1:1 propensity score matching on ten clinical variables (21) (manuscript in preparation).
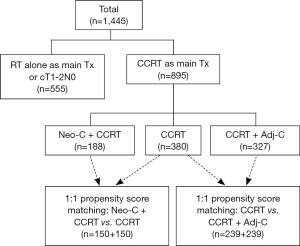
Comparisons between Neo-C + CCRT and CCRT were done on 300 patients (150 from each group) (21) and revealed that there was no advantage by adding Neo-C with respects to the clinical outcomes of 5-year OS (81.1% vs. 72.1%, P=0.340), DF survival (57.1% vs. 52.2%, P=0.978), and distant metastasis-free survival (76.2% vs. 64.9%, P=0.390). LR control at 5 years, however, was significantly worse in Neo-C + CCRT group (72.4% vs. 85.2%, P=0.014), which might have been related to lengthier RT duration following Neo-C. Based on these findings, Neo-C should not be routinely recommended to all patients but should only be considered in the limited patients in whom tumor volume reduction before CCRT would be desired (Ex: tumors that have invaded the skull base and are very close to the central nervous system).
Comparisons between CCRT and CCRT + Adj-C were done on 478 patients (239 from each group) (manuscript in preparation) and revealed that the addition of Adj-C improved DF survival at 5 years (71.3% vs. 60.3%, P=0.018), mainly by decreasing distant metastasis, but failed to improve OS (76.2% vs. 69.5%, P=0.089) and LR control (85.4% vs. 81.2%, P=0.112). Though Adj-C was able to reduce distant metastasis, its role on LR control and OS benefit is yet to be determined.
Weekly vs. triweekly chemotherapy in CCRT setting
Lee et al. (22) and Loong et al. (23) reported that higher cisplatin dose during CCRT could significantly improve LR control and survival. In CCRT setting, cisplatin chemotherapy typically was delivered at 3 weeks’ interval, which was usually associated with toxicities. Updated meta-analysis of 19 trials including 4,806 patients reported that CCRT significantly improved OS (24), and though not directly compared, either weekly cisplatin (30–40 mg/m2) or triweekly high-dose cisplatin (100 mg/m2) was thought to be similarly effective. Korea Cancer Study Group (25) conducted a multicenter randomized phase II study to compare triweekly cisplatin (3 doses of 100 mg/m2) and weekly cisplatin (7 doses of 40 mg/m2) and evaluated the efficacy and toxicity profiles. Analyses on 109 eligible patients from 19 Korean institutes, accrued from 1996 till 2012, demonstrated no differences with respects to DF survival and grade 3–4 toxicity profiles, while improved functional outcomes at 3 weeks following weekly regimen was achieved. Though weekly chemotherapy regimen, however, is not widely applicable in Korea because of the limitation by health insurance reimbursement policy, could be a reasonable option to the patients showing poor tolerance to high dose infusion by triweekly regimen.
Issues on RT detail
As stated above, advances in RT technique in treating NPCa, from 2DRT to 3DCRT to IMRT, were evident over time in Korea through the patterns of care study (7). OS improvement was evident during the third period (Table 1), when IMRT application was remarkably increased. This improvement, of course, coincided with the improved anatomic staging as well as the optimized combination of systemic chemotherapy with RT. The existence of large variations, however, was demonstrated through the plan comparison on two example cases by five departments in the delineations of target and normal organs and the dose schedules (26). These mainly physician-related factors could have influenced the quality of IMRT plans and the consequent clinical outcomes. No single standard recommendation on target definition, dose constraints to the normal organs and dose fractionation schedule has been available as of yet in Korea.
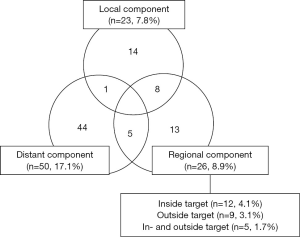
Bilateral entire neck irradiation has long been traditionally recommended. There have been, however, increasing evidence supporting selective neck irradiation (SNI) concept in treating NPCa. Two target volumes are defined with references to all clinical information at Samsung Medical Center: gross tumor volume (GTV) is defined as grossly visible and known primary and metastatic lesions; and clinical target volume (CTV) is defined as the tissues immediately adjacent to GTV and lymph nodes at equivocal risk of metastasis. SNI policy was applied to 293 consecutive NPCa patients from January 2001 till July 2013 and differential dose schedules were delivered to GTV and CTV by serial shrinking field with or without simultaneous boost technique (27). After the median follow-up duration of 56 months, 85 patients (29.0%) developed treatment failures and distant metastasis component was most common in 50 (17.1%), followed by regional failure in 26 (8.9%) and local failure in 23 (7.8%), respectively (Figure 2). Among those with regional relapse component, 12 patients (4.1%) developed inside CTV recurrence, nine (3.1%) did outside recurrence, and five (1.7%) did both inside and outside recurrences, respectively. Lower neck lymphatic(s) was (were) not irradiated in 143 patients (48.8%) based on the above target definition, and only four (2.8%) developed recurrence in the un-irradiated lower neck. Only one patient (0.3%) developed level 1B recurrence. Based on these very low rates of outside target recurrence following target delineation with careful evaluation of all clinical information, SNI policy seemed highly reasonable strategy in terms of effectiveness and safety.
Predictive nomogram
Anatomic staging system only sometimes is not sufficient in predicting the clinical outcomes. Individual patients’ data of 270 NPCa patients, who were given RT or CCRT at Samsung Medical Center from 1996 till 2012, were analyzed to identify the important factors on response to RT and OS (28). Nomograms were created based on the prognostic factors and external validation was attempted using the dataset of 122 patients, who were treated at Pusan National University Hospital from 1994 till 2009. This validation showed perfect correlation with each other, and utilization of these nomograms in personalized care and counselling would be anticipated.
Summary
Korea is located in non-endemic region of EBV infection and the incidence rate of NPCa is quite lower when compared with that in other parts of Asian continent. Korean NPCa patients, though very different in epidemiologic point, have similar clinical and histologic characteristics (EBV positivity and frequency of non-keratinizing subtype) to those in EBV infection endemic regions. KROG established a database of NPCa patients, who were treated from 1988 till 2011, and a few informative studies were performed based on the database (treatment patterns and clinical outcomes of cN0 patients, role of chemotherapy in stage II patients, role of Neo-C and Adj-C added to CCRT in patients with locoregionally advanced stages). It was evident that the clinical outcomes improved along the time frame, which was the contribution not only of improved anatomic staging and RT technique, but also of optimal use of systemic chemotherapy. Important findings from KROG database and KCSG prospective trial mostly have conformed to those from the previously reports by others (retrospective, prospective, and meta-analysis). Large variation with respects to the detail in IMRT planning was recognized and the key principles currently applied at Samsung Medical Center were briefly introduced with promising outcomes. Based on the current understandings of Korean perspective in management of NPCa, future refinement aiming at improved clinical outcomes through large scale prospective clinical trial would be anticipated.
Acknowledgements
Authors would express our deep appreciation to all members of Head/Neck Cancer subcommittee of Korean Radiation Oncology Group (KROG), who participated in the development of the KROG 11-09 database and subsequent studies and publications. This manuscript could be successfully completed with Dr. Ha Kyoung Kim’s enthusiastic assistance in formatting the references.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Annual report of cancer statistics in Korea in 2013, Ministry of Health and Welfare, 2016. Available online: http://www.cancer.go.kr/mbs/cancer/subview.jsp?id=cancer_040101000000. In Korean.
- Lee JH, Shin SW. Overdiagnosis and screening for thyroid cancer in Korea. Lancet 2014;384:1848. [Crossref] [PubMed]
- Ho JH. An epidemiologic and clinical study of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 1978;4:182-98. [Crossref] [PubMed]
- Jeon YK, Lee BY, Kim JE, et al. Molecular characterization of Epstein-Barr virus and oncoprotein expression in nasopharyngeal carcinoma in Korea. Head Neck 2004;26:573-83. [Crossref] [PubMed]
- Kim YJ, Go H, Wu HG, et al. Immunohistochemical study identifying prognostic biomolecular markers in nasopharyngeal carcinoma treated by radiotherapy. Head Neck 2011;33:1458-66. [Crossref] [PubMed]
- NCCN clinical practice guidelines in oncology: Head and Neck cancers, 2016. Available online: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- Sung SY, Kang MK, Kay CS, et al. Patterns of care for patients with nasopharyngeal carcinoma (KROG 11-06) in South Korea. Radiat Oncol J 2015;33:188-97. [Crossref] [PubMed]
- Park H, Ahn YC, Oh D, et al. Treatment outcomes of N0 stage nasopharyngeal carcinoma: a multi-institutional study. The 31st annual meeting of KOSRO (Meeting abstract), October 2013.
- Kang MK, Oh D, Cho KH, et al. Role of Chemotherapy in Stage II Nasopharyngeal Carcinoma Treated with Curative Radiotherapy. Cancer Res Treat 2015;47:871-8. [Crossref] [PubMed]
- Bachouchi M, Cvitkovic E, Azli N, et al. High complete response in advanced nasopharyngeal carcinoma with bleomycin, epirubicin, and cisplatin before radiotherapy. J Natl Cancer Inst 1990;82:616-20. [Crossref] [PubMed]
- Atichartakarn V, Kraiphibul P, Clongsusuek P, et al. Nasopharyngeal carcinoma: result of treatment with cis-diamminedichloroplatinum II, 5 fluorouracil, and radiation therapy. Int J Radiat Oncol Biol Phys 1988;14:461-9. [Crossref] [PubMed]
- Clark JR, Norris CM Jr, Dreyfuss AI, et al. Nasopharyngeal carcinoma: the Dana-Farber Cancer Institute experience with 24 patients treated with induction chemotherapy and radiotherapy. Ann Otol Rhinol Laryngol 1987;96:608-14. [Crossref] [PubMed]
- Chan AT, Teo PM, Leung TW, et al. A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 1995;33:569-77. [Crossref] [PubMed]
- Chua DT, Ma J, Sham JS, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol 2005;23:1118-24. [Crossref] [PubMed]
- Ma J, Mai HQ, Hong MH, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol 2001;19:1350-7. [PubMed]
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [PubMed]
- Chi KH, Chang YC, Guo WY, et al. A phase III study of adjuvant chemotherapy in advanced nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys 2002;52:1238-44. [Crossref] [PubMed]
- Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012;13:163-71. [Crossref] [PubMed]
- Yan M, Kumachev A, Siu LL, et al. Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: A Bayesian network meta-analysis. Eur J Cancer 2015;51:1570-9. [Crossref] [PubMed]
- Song JH, Wu HG, Keam BS, et al. The Role of Neoadjuvant Chemotherapy in the Treatment of Nasopharyngeal Carcinoma: A Multi-Institutional Retrospective Study Using Propensity Score Matching Analysis. Cancer Res Treat 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer 2011;47:656-66. [Crossref] [PubMed]
- Loong HH, Ma BB, Leung SF, et al. Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother Oncol 2012;104:300-4. [Crossref] [PubMed]
- Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015;16:645-55. [Crossref] [PubMed]
- Lee JY, Sun JM, Oh DR, et al. Comparison of weekly versus triweekly cisplatin delivered concurrently with radiation therapy in patients with locally advanced nasopharyngeal cancer: A multicenter randomized phase II trial (KCSG-HN10-02). Radiother Oncol 2016;118:244-50. [PubMed]
- Park SH, Park HC, Park SW, et al. Multi-institutional comparison of intensity modulated radiation therapy (IMRT) planning strategies and planning results for nasopharyngeal cancer. J Korean Med Sci 2009;24:248-55. [Crossref] [PubMed]
- Lee E, Ahn YC, Oh D, et al. Patterns of regional failure after selective neck irradiation in nasopharyngeal carcinoma patients. The 32nd annual meeting of KOSRO (Meeting abstract), October 2014.
- Cho JK, Lee GJ, Yi KI, et al. Development and external validation of nomograms predictive of response to radiation therapy and overall survival in nasopharyngeal cancer patients. Eur J Cancer 2015;51:1303-11. [Crossref] [PubMed]