Therapeutic vaccination strategies to treat nasopharyngeal carcinoma
Epstein-Barr virus (EBV): co-existence and disease
EBV, one of eight human herpesviruses, infects over 95% of people worldwide (1). EBV is transmitted orally and is often acquired during childhood, the infection going unnoticed or at least not standing out from the usual minor infections that occur in children. In more socioeconomically developed communities infection with EBV is often delayed until adolescence. Here infection may also pass unnoticed although some people develop infectious mononucleosis, a transient but debilitating condition characterised by symptoms of sore throat, lymphoadenopathy, fever and fatigue (2). In both cases EBV infection is countered by a robust immune response comprising natural killer cells, CD8+ cytotoxic T-cells and CD4+ helper T-cells (3). This response can be extremely strong and the symptoms of infectious mononucleosis are thought to stem from an over-exuberant immune response to infection. Nevertheless EBV establishes permanent colonisation of a subset of the host’s B lymphocytes, evading anti-viral immunity by silencing viral protein expression. This strategy enables EBV to persist for the lifetime of the host as a latent infection, a hallmark of the herpesvirus family. In order to be transmitted to new hosts, some of the B cells carrying EBV undergo reactivation, producing new progeny virus in the oropharynx for transmission to susceptible individuals (4).
The fine balance that exists between EBV and its host means that most people suffer no long-term health effects from this infection. Although relatively benign in most people, EBV has powerful growth transforming potential and is classified as a group I carcinogen by the World Health Organisation (5). It is aetiologically linked to several distinct lymphomas: Burkitt lymphoma, Hodgkin lymphoma, extranodal T/NK lymphoma (ENKTL) and an estimated 10% of diffuse large B cell lymphomas (5). EBV is also a problem in the transplant setting, where iatrogenic immunosuppression of normal anti-viral immunity may result in posttransplant lymphoproliferative disease (PTLD) (3,4). The largest burden of disease, however, stems from EBV’s ability to infect and transform epithelial cells. EBV is associated with 10% of gastric carcinomas and almost all cases of the non-keratinising subtype of nasopharyngeal carcinoma (NPC) (5). This subtype represents most cases (>95%) of NPC in geographical regions where the disease is endemic. Taken together EBV is associated with an estimated 200,000 cases of cancer each year, representing 1% of all cancers worldwide (6).
All EBV-associated malignancies express viral proteins although the number of proteins varies for different diseases (Table 1). Post-transplant lymphomas, particularly those that arise in the first year following transplantation when immunosuppression is greatest, express all eight EBV latent cycle proteins. These comprise six Epstein-Barr Nuclear Antigens (EBNAs) and two Latent Membrane Proteins (LMPs). Notably the EBNA 3A, 3B and 3C proteins, which are particularly good CD8+ T-cell targets, are expressed (4). In contrast, a more limited range of less immunogenic EBV proteins are expressed in the other EBV-associated malignancies, possibly reflecting their origin in people who are not overtly immunosuppressed.
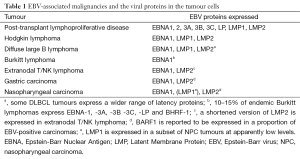
Full table
Several clinical trials of immunotherapies targeting these EBV proteins have been conducted worldwide. Most work to date has focussed on adoptive T-cell therapy, with T-cell effectors being prepared in vitro for infusion into patients (7-12). This approach has been applied mostly to PTL although several clinical trials have extended it to other EBV-associated lymphomas (13-15) and to NPC (16-23). A smaller number of trials have employed a different strategy, using therapeutic vaccines to boost relevant T-cell responses in the patient themselves (24-27). Finally, interim results have recently been released for a clinical trial in NPC patients of an immune checkpoint antagonist targeting Protein Death receptor 1 (PD1) (28), an approach that could potentially be used by itself but with considerable potential for synergy when combined with the aforementioned therapies.
Tumor associated antigens as targets for immunotherapy
A large number of tumor-associated antigens (TAAs) have been identified. They can be divided into two broad groups based on their expression pattern (29). Some have low tumoral specificity. Overexpressed TAAs, for example, are present at high levels in cancerous cells but lower levels in healthy tissue. Others have much higher tumoral specificity making them more attractive targets for immunotherapy. In this latter group are neoantigens, altered proteins generated through cancer-specific somatic mutation. These are exquisitely tumor specific allowing the cancer to be targeted with minimal risk of damage to normal tissues (30). Because they arise after later in life, after the immune system has developed, neoantigens represent foreign immune targets. Neoantigen-specific T-cells are therefore highly avid because they have not been subjected to central tolerance (30). Neoantigen generation is a stochastic process requiring a mutation that: (I) is located in the open reading frame of a gene expressed by the cancer cell; (II) generates an altered peptide capable of being presented by one of the patient’s HLA molecules; and (III) is able to stimulate a cognate T-cell response (31,32). These requirements mean that neoantigens are more likely to occur in cancers with high mutational load, such as melanoma and smoking-associated lung cancer, and less likely in others (33-35).
The mutational load of the EBV-associated malignancies is relatively low, reducing the probability of exploitable neoantigens being present in a patient’s tumor (36-38). In principle, however, the virus-encoded proteins expressed in the malignant cells of the EBV associated cancers might also serve as neoantigens. Immune responses to these EBV-encoded antigens are likely to be tumor selective because the viral proteins are otherwise expressed in a very limited number of EBV-infected B cells. It is also reasonable to expect that EBV-specific T-cells have not undergone central tolerance; the high avidities exhibited by EBV-specific T-cell clones in vitro suggests this is indeed the case (39-45). Unlike many mutational neoantigens, which are ‘private’ and limited to only a single patient’s cancer, all EBV-positive tumors express one or more viral proteins and immune therapies targeting them therefore have wide applicability.
Epstein-Barr virus (EBV)-encoded immunotherapeutic targets in nasopharyngeal carcinoma (NPC)
Two EBV proteins are consistently expressed in NPC. The first is EBNA1, a protein of critical importance to the virus as it maintains the viral DNA in dividing cells. EBNA1 also regulates the expression of viral and cellular genes, modulating a range of cellular pathways (46). EBNA1 contains a large glycine/alanine repeat domain that interferes with the protein’s processing and presentation by the HLA-class I antigen processing pathway. Although this reduces EBNA1’s visibility to CD8+ T-cells, it is important to note that EBNA1-positive cells can still be recognised by these effectors (47-49). EBNA1 possesses many more CD4 T-cell epitopes and T-cell responses to these are frequently detected in the population (50,51). EBNA1 was the first viral protein identified as being processed by macroautophagy and some, but not all, CD4+ T-cell epitopes are generated by this pathway (52-54). Such endogenous processing is important because it could potentially allow EBNA1-specific CD4 T-cells to act as direct effectors against HLA-class II positive cells and in this respect it is interesting to note that NPC tumor cells are often HLA-class II positive (55,56). Although EBNA1’s nuclear localisation decreases its processing by macroautophagy and consequently visibility to CD4+ T-cells (53), EBNA1-specific CD4+ T-cells suppressed tumor growth in an animal model of Burkitt Lymphoma (57). The recent report of clinical responses in PTL patients treated with EBNA1-stimulated T-cell lines (7) also supports harnessing EBNA1 as an immunotherapeutic target.
The second protein consistently expressed in NPC is the transmembrane protein LMP2. Although its principle function is to negatively regulate B-cell receptor signalling, LMP2 exhibits a range of activities and is required for the outgrowth of epithelial cells in vitro (58). LMP2 mRNA transcripts are detected in more than 98% of NPC biopsies and protein can be detected by immunohistochemistry in >50%; this discrepancy likely being due to the lower sensitivity of the latter technique (59). LMP2 contains many CD8+ T-cell epitopes (4,39,41,42,60) and is therefore considered the best CD8+ T-cell target expressed in NPC. A smaller number of CD4 T-cell epitopes have also been identified in LMP2 and there is evidence that these epitopes are displayed by LMP2-positive cells as well (43). In LCLs LMP2 reduces expression of the co-activatory ligand NKG2D, decreasing T-cell recognition (61); it is not known if LMP2 has a similar effect in NPC cells.
Another membrane protein, LMP1, is present in a minority of NPC biopsies and appears to be expressed at lower levels in the tumor cells (62). LMP1 functions as a constitutively active member of the TNFR superfamily to provide growth and differentiation signals to B cell but exerts a range of effects in cells including the upregulation of anti-apoptotic proteins and is considered to be the main transforming protein of EBV (58). Although several CD8+ T-cell epitopes have been identified in LMP1 (40,60), this protein’s tendency to self-aggregate limits HLA-class I restricted LMP1 epitope generation (63). Several LMP1-specific CD4+ T-cell epitopes have been defined (43,44,51) and, as is the case for EBNA1 and LMP2, some of these epitopes are displayed on the surface of LMP1-positive cells (43). The antigen processing pathways involved in generating HLA-class II restricted LMP1 and LMP2 epitopes are currently unknown.
Although not expressed in lymphoid malignancies, the BARF1 protein is consistently detected in most EBV-positive NPC and gastric tumors (64,65). BARF1 is reported to have transforming activity, inhibit apoptosis and act as a colony stimulating factor 1 decoy receptor. Relatively little attention has been given to BARF1 as a T-cell target. Both CD8 and CD4 T-cell responses were detected in the blood of NPC patients and were present at lower levels in healthy donors (66). A number of HLA-A2 restricted CD8+T-cell epitopes have been defined (66) and, given the above result, it is likely that more remain to be discovered.
The immune status of nasopharyngeal carcinoma (NPC) patients
Given that NPC occurs in people who are not undergoing immunosuppressive therapy, an obvious but important question is whether they have suffered selective loss of immune responses to the EBV proteins present in the tumor. A comprehensive screen of newly diagnosed untreated NPC patients found that, with the exception of a HLA-B*4001 restricted LMP2-specific response, CD8+ and CD4+ T-cells responses against the EBNA1, LMP1 or LMP2 proteins were similar to those in healthy control donors (67). This work examined T-cell responses using ex vivo ELIspot assays that detect T-cells with immediate effector function, typically the effector memory subset. Studies using methods that detect both effector and central memory T-cells differ in their conclusions. Analysis by tetramer staining shows that newly diagnosed NPC patients still possess LMP1 and LMP2-specific T-cell responses although at lower frequencies compared to healthy donors (68). One study using in vitro culture methods reported that LMP2-specific T-cell responses appear normal at the time of diagnosis (69) whereas others report that LMP2 and EBNA1-specific CD8+ T-cells are decreased in frequency (70,71). Removing regulatory T-cells from the cultures increased the number of EBNA1 and LMP2-specific T-cells detected suggesting that regulatory T-cells may limit EBV-specific immunity (71). In this respect it is interesting to note that the frequency of regulatory CD4+ T-cells is raised in the blood of newly-diagnosed patients and those who have completed first-line chemoradiotherapy (24,72). Adjuvant chemotherapy with cisplatin has been reported to increase the frequency of regulatory T-cells in head and neck squamous cell carcinoma patients (73). It is not known whether this agent, the standard first-line chemotherapy for NPC, might similarly affect regulatory T-cell numbers in NPC patients.
Nasopharyngeal carcinoma (NPC) as a target for immune responses
The fact that many NPC patients still possess relevant EBV specific T-cell responses raises the question of whether these T-cells are capable of acting in the tumor itself. Early studies found that NPC cell lines express HLA class I molecules, possess normal antigen processing activity and, when tested in vitro, can be recognised and killed by T-cells (69,74). Analysis of NPC tumor biopsies shows that HLA class I is expressed in 63%, reduced in 22% and lost in 15% of cases (56). HLA class II is also frequently detected in tumours (55,56), raising the possibility that CD4+ T-cells may be able to play a direct anti-tumor role: indeed, such cells may be the only effective T-cell population in cases that have lost HLA class I expression.
The fact that HLA loss is uncommon in NPC may reflect the presence of other immune evasion mechanisms. The tumor cells themselves express multiple immunomodulatory molecules. These include HLA-G, an inhibitor of T and NK cell function that is associated with decreased survival (75) and galectin-9, which is secreted in exosomes and inhibits T-cell function (76). NPC is also characterised by a heavy infiltrate of lymphoid cells that may also exert suppressive effects. The infiltrating cells are predominantly CD3+ T-cells with smaller numbers of NK cells, B cells, dendritic cells (DCs) and monocytes detected (77). Regulatory T-cells are enriched in the tumor compared with adjacent normal nasopharyngeal tissue (72,78). Indoleamine 2,3-dioxygenase, which indirectly suppresses T-cell activity via tryptophan depletion, is present in 75% of tumor biopsies although it is currently unclear whether this represents expression by infiltrating myeloid derived suppressor cells or by the tumor cells (79). Although present in high numbers, the functional capacity of CD3+ T-cells in the tumor remains unclear. In 20% of NPC tumors they show reduced expression of the T-cell receptor zeta chain required for T-cell activation following target recognition (78). The fact that functional T-cells can be cultured from NPC tumors suggests that any impairment in T-cell function is potentially reversible (23,69).
The negative immune regulator PD-L1 is expressed in 89–95% of NPC tumors (28,80,81). This frequent expression provides an important therapeutic opportunity as several antibody-based therapies that can disable this inhibitory pathway are now licensed or in the advanced stages of clinical development (82). In some cases PD-L1 expression in the tumor is induced by inflammatory cytokines released by infiltrating immune cells and its expression here is thought to counter an anti-tumor immune response (83). Such cases may respond more favourably when PD-1 or PD-L1 inhibitors are used as monotherapy (84) as anti-tumor effectors are already present. An alternative, but not necessarily mutually exclusive, mechanism responsible for PD-L1 expression is intrinsic resistance. Here PD-L1 upregulation is the result of alterations in signalling pathways within the malignant cells or genetic amplification of the PD-L1 locus: the latter frequently occurs in EBV-associated gastric cancer (38). It is conceivable that such cases might be more likely to benefit from PD1 or PD-L1 inhibition when it is combined with immunotherapies capable of generating the tumor-specific immune responses that would otherwise be absent.
For NPC it is currently unclear which of these two mechanisms is responsible for the frequent expression of PD-L1. LMP1-mediated up-regulation of the AP-1, STAT3 or NF-kB pathways has been suggested to be responsible (80,81) and in this respect it is interesting to note that BL tumors, which lack LMP1, do not express PD-L1 (80). Conversely, the heavy lymphoid infiltrate is consistent with a role for adaptive immune resistance and this is supported by in vitro data that show interferon-gamma increases PD-L1 expression by NPC cell lines (81). Although the underlying mechanism of PD-L1 expression is unclear, interim data from KEYNOTE-028, a non-randomised Phase IB trial (NCT02054806) of the PD-1 inhibitor pembrolizumab in solid tumors, have provided a signal of efficacy for checkpoint blockade monotherapy in NPC. Of the 27 patients with advanced unresectable or metastatic NPC treated with pembrolizumab, one experienced a complete response, six experienced partial responses and 14 had stable disease giving an overall response rate of 25.9% (28). These preliminary data are encouraging and, given that PD1 inhibitors are generally well tolerated, there is clearly potential for them to be used in combination with other agents to increase response rates.
Lessons from infusional T-cell therapy for Epstein-Barr virus (EBV)-positive cancers
In the laboratory EBV readily infects and transforms B cells into permanently growing lymphoblastoid cell lines (LCLs). These B-cell lines, like PTL tumors, express all eight latent cycle proteins, high levels of HLA class I and class II molecules and they efficiently activate and expand EBV-specific T-cells in vitro. The specificity of the resulting T-cell lines tend to focus on the immunodominant EBNA3A, 3B and 3C proteins. Because PTLD tumors express these proteins infusion of such T-cell lines has proven highly successful as prophylaxis and therapy for PTLD in the haematological transplant setting (8) with evidence of efficacy for PTLD following solid organ transplantation (9). LCLs have also been used to establish T-cell banks from third party donors and these have also shown efficacy when used to treat partly HLA-matched PTLD patients (10). Subsequent studies have extended adoptive T-cell therapy to patients with other EBV-associated lymphomas (85). The T-cell lines used in these trials were initially generated using LCLs, with more recent trials employing a range of techniques to focus the immune response onto the smaller number of EBV antigens present in the malignant cells of Hodgin Lymphoma and ENKTL (13-15). Detailed discussion of these trials is beyond the scope of this review, and we will instead highlight four key insights from this work relevant for NPC immunotherapy.
First, focusing the EBV-specific immune response onto a limited repertoire of epitopes runs the risk of the tumor escaping this narrow immunological pressure. A PTLD patient’s lack of response to T-cell therapy was discovered to be caused by the tumor, which expressed a truncated EBNA3B protein, being poorly recognised by the infused T-cells. The T-cell line, prepared using LCLs as the antigenic stimulus, was dominated by T-cells specific for two epitopes located in the region of EBNA3B that was lost from the tumor cells (86). This incident may represent a special case, with high level immunosuppression allowing the proliferation of tumor cells carrying this unusual mutation, but it clearly illustrates the dangers inherent in relying on a highly focussed immune response which could become a single point of failure. Using a diverse range of T-cell responses to provide redundancy and targeting proteins the malignant cells needs for cellular growth or survival will both help minimise the risk of treatment failure.
Second, LCL-stimulated T-cell lines containing high numbers of CD4 T-cells yielded significantly better clinical responses in PTLD patients (87). Whether this is due to these cells acting as effectors in their own right, providing help to CD8 T-cells or a combination of the two is unknown, but it adds further support for using a broad range of EBV-specific effectors in patients.
Third, EBV specific T-cells are able to induce clinical responses in patients with Hodgkin Lymphoma, a disease with a complex immunosuppressive tumor microenvironment and, in clinical trials, T-cell preparations enriched for LMP2-specific T-cells are more effective (13-15).
Fourth, EBNA1-specific T-cells yielded clinical responses in seven of ten PTLD patients treated with them. These clinical responses were associated with expansion of the infused EBNA1-specific T-cells in vivo (7). Increases in LMP2-specific T-cell responses were also detected in three donors and although these could indicate contaminating cells in the infused T-cell product, they could equally represent antigen spreading stimulated by the release of antigen from lysed tumor cells. Overall, this study suggests EBNA1 should be given serious consideration as a potential target in future therapeutic strategies.
Treating NPC with infusional T-cell therapy represents a much greater challenge because of the more limited range of EBV targets in the tumor cells and, as described above, local and systemic immunosuppressive mechanisms need to be overcome. Adoptive T-cell therapy for NPC is discussed elsewhere in this special issue of Chinese Clinical Oncology and we will therefore discuss this work only briefly. A number of groups have used LCL-stimulated autologous T-cell lines to treat NPC (16-20,88,89) and some studies have reported clinical responses. In a Phase I trial of ten patients with stage IV NPC in progression after chemotherapy and radiotherapy, two partial responses and four cases of stable disease were observed after T-cell infusion (18). A separate Phase I/II clinical study observed complete responses in three of four patients with locoregional disease but only one of eleven patients with metastatic disease (19,42). Administering lymphodepleting chemotherapy or antibodies prior to T-cell therapy, to promote greater T-cell expansion in vivo, did not markedly increase the number of clinical responses observed (16,20). These trials each recruited a mixed cohort of patients, many of whom had undergone multiple lines of treatment. To explore the efficacy of adoptive T-cell therapy as first line therapy, 35 newly diagnosed NPC patients were treated with four cycles of gemcitabine and carboplatin before receiving autologous LCL-stimulated T-cells (21). After T-cell infusion thirteen patients had a further partial response and seven had stable disease.
Two groups have used alternative methods to prepare therapeutic T-cells for infusion. To focus the immune response against the subset of EBV antigens expressed in NPC, Smith and colleagues prepared T-cell lines using as the antigen stimulus an adenovirus expressing a fusion protein of EBNA1 (lacking the glycine/alanine repeat domain) and a polyepitope string of defined LMP1 and LMP2 peptides (22). Although clinical responses were not observed, median overall survival for the patients treated with T-cells, who had locoregional recurrence or distant metastases, was greater than a historical patient cohort. In a different study, patients were treated with a single dose of autologous TILs one week after completing chemoradiotherapy (23). The TILs had a high frequency of CD4+ T-cells and consistently responded to EBNA1 peptides when tested in vitro. Following TIL infusion, increases in LMP1, LMP2 and EBNA1-specific T-cells could be detected in the blood of some patients. The near-contemporaneous use of chemotherapy and TILs in this trial makes it difficult to determine whether T-cell infusion resulted in improved outcomes compared to chemotherapy alone. This question may be addressed by a Phase II trial that is reported to be underway (23).
Autologous dendritic cell vaccination for nasopharyngeal carcinoma (NPC)
The first therapeutic vaccination trial for NPC consisted of four cycles of autologous monocyte-derived DCs loaded with LMP2 CD8+ T-cell epitope peptides (26). All 16 patients had residual disease when recruited to the study and LMP2-specific T-cell responses were very low or undetectable in ex vivo ELIspot assays. Vaccination boosted LMP2-specific T-cells in nine patients and these increases were sustained for three months before declining. Seven patients failed to make a T-cell response of whom four had persistent leukopaenia and immune impairment on trial entry. Partial clinical responses were detected in two patients coincident with increases in circulating LMP2-specific T-cell frequency.
A trial of similar design, again using autologous DCs loaded with LMP2 CD8+ T-cell epitope peptides, showed vaccination increased circulating LMP2-specific T-cells in 7 of 16 patients treated (23). Clinical responses were not described but a small decrease in serum EBV DNA levels, a surrogate marker of tumor burden, was noted. This decrease did not correlate with immune responses in the blood but instead correlated with the presence of a delayed type hypersensitivity response to the LMP2 peptides used to immunize each patient.
Both of the above studies used a small number of defined LMP2 CD8+ T-cell epitope peptides selected on the basis of the patients HLA type. An alternative approach by Chia and colleagues used autologous DCs transduced with an adenovirus expressing truncated LMP1 and full length LMP2 protein (27). This method has the advantage of potentially boosting a wider range of T-cell specificities including those that are currently undefined or are presented through rare HLA alleles. Patients recruited to the study had been heavily pre-treated and on trial entry only one had a detectable T-cell response to LMP1, LMP2 or EBNA1 in ex vivo ELIspot assays. Following vaccination, 9 of 12 patients showed increased delayed type hypersensitivity reactions to transduced DCs. No increases in T-cell responses were detected by ex vivo ELIspot assays but, nevertheless, one partial clinical response and two instances of stable disease were achieved.
Non-cellular therapeutic vaccination for nasopharyngeal carcinoma (NPC)
The above studies are important as they show vaccination can overcome any systemic immunosuppression that may exist in NPC patients to boost therapeutically relevant T-cell responses. Customized patient-specific DC vaccines, however, require highly trained staff and specialized facilities presenting a significant challenge to their widespread use. A vaccine that could be mass-produced would be far better suited to widespread use, particularly in countries with limited health resources. The economic case for such an ‘off-the-shelf’ vaccine is compelling. A low marginal cost of production means it would benefit from economies of scale, with the cost per dose decreasing as larger amounts of vaccine are made. This fundamental economic concept led us to develop an off-the-shelf therapeutic vaccine to treat NPC or indeed any other EBV-associated cancer. Based on the modified vaccinia Ankara (MVA) vector, which is highly attenuated and has an excellent clinical safety record (90), MVA-EBNA1/LMP2 encodes a fusion protein consisting of the carboxy terminal half of EBNA1 fused to full length LMP2 (91). This design retains almost all known CD4 T-cell epitopes and removes the EBNA1 glycine/alanine repeat domain that would otherwise interfere with protein expression and antigenic processing. All known LMP2 CD8 and CD4 T-cell epitopes are included in the construct and its antigenicity is enhanced by the LMP2 protein sequence redirecting EBNA1 into the HLA class II processing pathway (91).
Two dose escalation Phase IA trials of MVA-EBNA1/LMP2, conducted in NPC patients in Hong Kong and the United Kingdom, have demonstrated the vaccine is safe and well tolerated (25). Side effects were predominantly grade 1, with seven instances of grade 2 and one transient grade 3 in the 34 patients who were vaccinated in these trials. Before vaccination, low-level T-cell responses specific for EBNA1 and LMP2 were detectable in most patients in ex vivo ELIspot assays. Compared to previous studies this level of pre-existing T-cell response is much higher and likely reflects the fact that most patients had undergone only one line of therapy before trial recruitment. Of the 27 patients for whom data were available, 18 and 12 showed a post-vaccination increase in T-cells specific for EBNA1 and LMP2 respectively. There was clear evidence of a dose effect and all patients who received dose level three (2×108 pfu at three-weekly intervals) responded to EBNA1, LMP2 or both. Analysis of the fine detail of these antigen-specific responses using a panel of epitope peptides revealed that vaccination boosted a broad range of CD8+ and CD4+ T-cell responses against LMP2 and EBNA1 respectively in patients of Chinese and European descent (24). The vaccine was therefore immunogenic across the natural variation that exists for HLA alleles and circulating EBV strains in different populations.
The size of the immune responses stimulated by the vaccine was sufficient to allow T-cell phenotyping to be performed by ex vivo flow cytometry. This revealed several key insights. First, assays measuring interferon gamma, the method most commonly used to monitor immune responses in NPC immunotherapy trials, will underestimate the true size of the immune response because only a minority of EBNA1- and LMP2-specific T-cells produce this cytokine.
Second, the quality of the patient’s EBNA1- and LMP2-specific T-cells, determined by the number of effector functions they exhibited, was lower compared with T-cells specific for the non-tumor EBV protein EBNA3A (24). These differences in immune quality may represent a wider phenomenon as they have also been observed in some, but not all, studies performed in healthy donors (92,93). When tested in vitro, galectin-1 suppressed the activity of low quality LMP-specific T-cells whereas high quality T-cells were resistant (92). Vaccination-induced increases in immune quality may therefore allow T-cells to overcome the array of immunosuppressive mechanisms present in NPC tumors.
Third, as might be expected LMP2-specific CD8+ T-cells and EBNA1-specific CD4+ T-cells possessed distinct functional properties. The former degranulated upon antigen stimulation, consistent with perforin-mediated cytotoxicity. Importantly, vaccination greatly increased the number of LMP2-specific CD8+ T-cells capable of degranulation when exposed to antigen. By contrast, EBNA1-specific CD4+ T-cells did not degranulate in response to stimulation. Note that this does not necessarily mean these cells lack cytotoxicity as they have reported to utilise the FAS/FAS-Ligand pathway (94). Many of the CD4+ T-cells were able to produce interleukin-2, a property the almost all LMP2-specific CD8 T-cells lacked. Taken together, these functional differences between the two immune subsets support our strategy of including both antigens in the vaccine.
Fourth, treating patients with three cycles of vaccination did not drive EBNA1 and LMP2-specific effectors to terminal differentiation, suggesting additional cycles of vaccination could be used to maintain or further boost these responses over time. Repeated monthly vaccinations have been used for a different poxvirus-based vaccine to maintain long-term control of pancreatic and rectal cancer (95).
These results have led to two subsequent clinical trials of MVA-EBNA1/LMP2. In the United Kingdom a Phase IB trial (NCT01800071) in NPC patients in remission or with current disease is examining vaccine immunogenicity in finer detail and testing the potential boosting effects of using a fourth vaccine cycle. In Hong Kong a Phase II trial (NCT01094405) is treating patients with persistent, recurrent or metastatic NPC to determine the clinical efficacy of the vaccine.
Looking to the future: increasing the efficacy of nasopharyngeal carcinoma (NPC) immunotherapy
As others and we have shown, it is possible to boost therapeutically relevant T-cells in NPC patients through adoptive T-cell therapy or vaccination. The challenge now is to improve the clinical response rate in NPC patients. One way to do this is to combine therapies. Some positive steps have already been taken in this direction (16,20,21) and there is enormous potential for further research in this area.
How would a therapeutic EBV vaccine help in this endeavour? First, a vaccine could complement adoptive T-cell NPC therapies of NPC. Vaccination following T-cell infusion could potentially boost the number of T-cells in the patient and sustain these increases for longer periods of time. Arguing against this strategy is the fact that the presence of a detectable T-cell response in the blood often does not correlate with clinical responses in NPC patients (19,22,89). It is important to note, however, that this lack of correlation does not mean that increasing the magnitude of the T-cell response is pointless. Rather, it may simply reflect the fact that only 2% of T-cells are present in the blood (96), the key effectors migrating to relevant tissues. Although chemokine receptors have been suggested as being involved in T-cell homing to NPC tumors (97,98), surprisingly little attention has been given to the homing properties of the T-cells used to treat NPC, whether generated in vitro for infusion or boosted in vivo via vaccination. With this in mind, one could envisage administering the vaccine via a route that would boost the number of T-cells but also encourage them to home to the relevant anatomical sites (99). In this respect it is interesting to note that an MVA tuberculosis vaccine (the same vector as MVA-EBNA1/LMP2) can be safely administered as an inhaled aerosol and this method of delivery boosted mucosal immunity (100). Modifying the vaccination route could be a simple way of achieving the best balance of circulating and tissue resident CD4+ and CD8+ T-cells to achieve control of locoregional and disseminated disease.
Second, there is increasing awareness that conventional cancer therapies may exert immune modifying effects. Radiotherapy can stimulate antigen processing and presentation pathways (101) and some chemotherapies and targeted therapies can affect the number and function of regulatory immune cells or alter the tumor microenvironment (102,103). Furthermore, an increasing number of small molecules (104) and biologic drugs (82) that manipulate the immune system are being developed or are already in clinical use. The cancer immunity cycle provides a useful conceptual framework for considering which agents to combine together (105). The induction of an effective anti-tumor immune response requires all of the rate limiting steps in the cycle to be overcome. For NPC the frequent expression of PD-L1 is likely to represents a fundamental rate-limiting step at a late stage in the cycle. The deficit in EBNA1 and LMP2-specific T-cells in NPC patients represents a second rate-limiting step operating at an early stage of the cycle (70,71). Contemporaneously removing both rate-limiting steps, by combining anti-PD1 with vaccination, could substantially increase clinical response in NPC patients as is the case for other settings (106-108). An important caveat here is that additional immunosuppressive mechanisms may also need to be addressed and these could potentially vary for different patients and stages of disease (109).
Third, despite radiotherapy and chemoradiotherapy some 5% to 15% of NPC patients develop local failure and 15% to 30% experience failure at distant sites with poor prognosis (110). An ‘off-the-shelf’ therapeutic vaccine, benefitting from economies of scale and therefore suitable for widespread use, could conceivably be used to vaccinate large numbers of patients following first line therapy to reduce the risk of recurrence. The advantage of this approach is two-fold. First, it would avoid the toxicities that have so far hampered the use of additional chemotherapies in this way (111). Second, it would utilise vaccination when it is likely to be most effective, in less heavily treated patients with low volume disease (112). NPC presents an ideal situation to test this strategy because (I) the same target EBV antigens are expressed in all tumors and (II) there is a simple and reliable way to stratify patients into risk groups. Patients who have elevated EBV plasma DNA after first line therapy have almost 12 fold greater risk of recurrence (113). If successful in these high risk patients, one could envisage vaccination being extended to all patients to eliminate occult micrometastases, resulting in further decreases in recurrence rates.
This review has focussed on the prospects for a therapeutic EBV vaccine that would benefit NPC patients in the immediate future. The success of prophylactic vaccines against hepatitis B virus and, more recently, oncogenic strains of human papillomavirus has stimulated interest in the possibility of primary prevention of the EBV-associated cancers (114). A small Phase II trial of a candidate EBV vaccine, based on the viral envelope glycoprotein gp350, reported a decrease in infectious mononucleosis but no overall protection against EBV acquisition. Although encouraging, a better immune response clearly needs to be induced to achieve complete protection. Multimeric forms of gp350 have now been developed and these have much stronger immunogenicity in animals compared to the monomeric gp350 used in the Phase II trial (115-117). Vaccine efficacy could also be improved by incorporating additional EBV glycoproteins and harnessing appropriate cellular immune responses (118,119). A safe, effective prophylactic EBV vaccine would be a major advance in human health. Its development is an important long-term goal, but it is also important to realize that the enormous number of people that already carry the virus, and for whom a preventative vaccine is already too late, means that NPC incidence will not decrease until years after a preventative vaccination campaign has started. Developing better ways to treat NPC will therefore remain an important area of research both now and in the future.
Acknowledgements
Work in GS Taylor’s laboratory is supported by research grants from Cancer Research UK, Bloodwise and the Gregor Mackay Memorial Fund.
Footnote
Conflicts of Interest: GS Taylor has been paid for developing and delivering educational presentations for Amgen and does consultancy work for Genocea Biosciences Inc. NM Steven has no conflicts of interest to declare.
References
- de-Thé G, Day NE, Geser A, et al. Sero-epidemiology of the Epstein-Barr virus: preliminary analysis of an international study - a review. IARC Sci Publ 1975.3-16. [PubMed]
- Balfour HH, Odumade OA, Schmeling DO, et al. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. J Infect Dis 2013;207:80-8. [Crossref] [PubMed]
- Rickinson AB, Long HM, Palendira U, et al. Cellular immune controls over Epstein-Barr virus infection: new lessons from the clinic and the laboratory. Trends Immunol 2014;35:159-69. [Crossref] [PubMed]
- Taylor GS, Long HM, Brooks JM, et al. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol 2015;33:787-821. [Crossref] [PubMed]
- IARC. Epstein-Barr virus. In: International Agency for Research on Cancer. A review of human carcinogens Part B: Biological agents. Lyon, France: WHO Press, 2012:49-80.
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006;118:3030-44. [Crossref] [PubMed]
- Icheva V, Kayser S, Wolff D, et al. Adoptive transfer of Epstein-Barr virus (EBV) nuclear antigen 1-specific T cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol 2013;31:39-48. [Crossref] [PubMed]
- Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010;115:925-35. [Crossref] [PubMed]
- Khanna R, Bell S, Sherritt M, et al. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci U S A 1999;96:10391-6. [Crossref] [PubMed]
- Haque T, McAulay KA, Kelly D, et al. Allogeneic T-cell therapy for Epstein-Barr virus-positive posttransplant lymphoproliferative disease: long-term follow-up. Transplantation 2010;90:93-4. [Crossref] [PubMed]
- Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol 2012;9:510-9. [Crossref] [PubMed]
- Barker JN, Doubrovina E, Sauter C, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood 2010;116:5045-9. [Crossref] [PubMed]
- Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol 2014;32:798-808. [Crossref] [PubMed]
- Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin’s disease. J Exp Med 2004;200:1623-33. [Crossref] [PubMed]
- Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood 2007;110:2838-45. [Crossref] [PubMed]
- Secondino S, Zecca M, Licitra L, et al. T-cell therapy for EBV-associated nasopharyngeal carcinoma: preparative lymphodepleting chemotherapy does not improve clinical results. Ann Oncol 2012;23:435-41. [Crossref] [PubMed]
- Chua D, Huang J, Zheng B, et al. Adoptive transfer of autologous Epstein-Barr virus-specific cytotoxic T cells for nasopharyngeal carcinoma. Int J Cancer 2001;94:73-80. [Crossref] [PubMed]
- Comoli P, Pedrazzoli P, Maccario R, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol 2005;23:8942-9. [Crossref] [PubMed]
- Louis CU, Straathof K, Bollard CM, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother 2010;33:983-90. [Crossref] [PubMed]
- Louis CU, Straathof K, Bollard CM, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood 2009;113:2442-50. [Crossref] [PubMed]
- Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther 2014;22:132-9. [Crossref] [PubMed]
- Smith C, Tsang J, Beagley L, et al. Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res 2012;72:1116-25. [Crossref] [PubMed]
- Li J, Chen QY, He J, et al. Phase I trial of adoptively transferred tumor-infiltrating lymphocyte immunotherapy following concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Oncoimmunology 2015;4:e976507. [Crossref] [PubMed]
- Taylor GS, Jia H, Harrington K, et al. A recombinant modified vaccinia ankara vaccine encoding Epstein-Barr Virus (EBV) target antigens: a phase I trial in UK patients with EBV-positive cancer. Clin Cancer Res 2014;20:5009-22. [Crossref] [PubMed]
- Hui EP, Taylor GS, Jia H, et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res 2013;73:1676-88. [Crossref] [PubMed]
- Lin CL, Lo WF, Lee TH, et al. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res 2002;62:6952-8. [PubMed]
- Chia WK, Wang WW, Teo M, et al. A phase II study evaluating the safety and efficacy of an adenovirus-ΔLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann Oncol 2012;23:997-1005. [Crossref] [PubMed]
- Hsu C, Lee S, Ejadi S, et al. Antitumor activity and safety of pembrolizumab in patients with PD-L1-positive nasopharyngeal carcinoma: Interim results from a phase 1b study. In European Journal of Cancer. London: Elsevier, 2015.
- Coulie PG, Van den Eynde BJ, van der Bruggen P, et al. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 2014;14:135-46. [Crossref] [PubMed]
- Lennerz V, Fatho M, Gentilini C, et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci U S A 2005;102:16013-8. [Crossref] [PubMed]
- Heemskerk B, Kvistborg P, Schumacher TN. The cancer antigenome. EMBO J 2013;32:194-203. [Crossref] [PubMed]
- Fritsch EF, Rajasagi M, Ott PA, et al. HLA-binding properties of tumor neoepitopes in humans. Cancer Immunol Res 2014;2:522-9. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- Yadav M, Jhunjhunwala S, Phung QT, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 2014;515:572-6. [Crossref] [PubMed]
- Lin D-C, Meng X, Hazawa M, et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genet 2014;46:866-71. [Crossref] [PubMed]
- Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet 2012;44:1321-5. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Lee SP, Thomas WA, Murray RJ, et al. HLA A2.1-restricted cytotoxic T cells recognizing a range of Epstein-Barr virus isolates through a defined epitope in latent membrane protein LMP2. J Virol 1993;67:7428-35. [PubMed]
- Khanna R, Burrows SR, Nicholls J, et al. Identification of cytotoxic T cell epitopes within Epstein-Barr virus (EBV) oncogene latent membrane protein 1 (LMP1): evidence for HLA A2 supertype-restricted immune recognition of EBV-infected cells by LMP1-specific cytotoxic T lymphocytes. Eur J Immunol 1998;28:451-8. [Crossref] [PubMed]
- Lee SP, Tierney RJ, Thomas WA, et al. Conserved CTL epitopes within EBV latent membrane protein 2: a potential target for CTL-based tumor therapy. J Immunol 1997;158:3325-34. [PubMed]
- Straathof KC, Leen AM, Buza EL, et al. Characterization of latent membrane protein 2 specificity in CTL lines from patients with EBV-positive nasopharyngeal carcinoma and lymphoma. J Immunol 2005;175:4137-47. [Crossref] [PubMed]
- Haigh TA, Lin X, Jia H, et al. EBV latent membrane proteins (LMPs) 1 and 2 as immunotherapeutic targets: LMP-specific CD4+ cytotoxic T cell recognition of EBV-transformed B cell lines. J Immunol 2008;180:1643-54. [Crossref] [PubMed]
- Kobayashi H, Nagato T, Takahara M, et al. Induction of EBV-latent membrane protein 1-specific MHC class II-restricted T-cell responses against natural killer lymphoma cells. Cancer Res 2008;68:901-8. [Crossref] [PubMed]
- Blake N, Lee S, Redchenko I, et al. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity 1997;7:791-802. [Crossref] [PubMed]
- Frappier L. EBNA1. Curr Top Microbiol Immunol 2015;391:3-34. [Crossref] [PubMed]
- Voo KS, Fu T, Wang HY, et al. Evidence for the presentation of major histocompatibility complex class I-restricted Epstein-Barr virus nuclear antigen 1 peptides to CD8+ T lymphocytes. J Exp Med 2004;199:459-70. [Crossref] [PubMed]
- Lee SP, Brooks JM, Al-Jarrah H, et al. CD8 T cell recognition of endogenously expressed Epstein-Barr virus nuclear antigen 1. J Exp Med 2004;199:1409-20. [Crossref] [PubMed]
- Tellam J, Connolly G, Green KJ, et al. Endogenous presentation of CD8+ T cell epitopes from Epstein-Barr virus-encoded nuclear antigen 1. J Exp Med 2004;199:1421-31. [Crossref] [PubMed]
- Bickham K, Münz C, Tsang ML, et al. EBNA1-specific CD4+ T cells in healthy carriers of Epstein-Barr virus are primarily Th1 in function. J Clin Invest 2001;107:121-30. [Crossref] [PubMed]
- Leen A, Meij P, Redchenko I, et al. Differential immunogenicity of Epstein-Barr virus latent-cycle proteins for human CD4(+) T-helper 1 responses. J Virol 2001;75:8649-59. [Crossref] [PubMed]
- Paludan C, Schmid D, Landthaler M, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 2005;307:593-6. [Crossref] [PubMed]
- Leung CS, Haigh TA, Mackay LK, et al. Nuclear location of an endogenously expressed antigen, EBNA1, restricts access to macroautophagy and the range of CD4 epitope display. Proc Natl Acad Sci U S A 2010;107:2165-70. [Crossref] [PubMed]
- Mautner J, Pich D, Nimmerjahn F, et al. Epstein-Barr virus nuclear antigen 1 evades direct immune recognition by CD4+ T helper cells. Eur J Immunol 2004;34:2500-9. [Crossref] [PubMed]
- Busson P, Braham K, Clausse B, et al. Constitutive expression of HLA class II antigens on EBV positive malignant cells from nasopharyngeal carcinoma: possible involvement in T cell infiltration. Cancer Detect Prev 1988;12:363-8. [PubMed]
- Yao Y, Minter HA, Chen X, et al. Heterogeneity of HLA and EBER expression in Epstein-Barr virus-associated nasopharyngeal carcinoma. Int J Cancer 2000;88:949-55. [Crossref] [PubMed]
- Fu T, Voo KS, Wang RF. Critical role of EBNA1-specific CD4+ T cells in the control of mouse Burkitt lymphoma in vivo. J Clin Invest 2004;114:542-50. [Crossref] [PubMed]
- Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol 2012;22:144-53. [Crossref] [PubMed]
- Heussinger N, Büttner M, Ott G, et al. Expression of the Epstein-Barr virus (EBV)-encoded latent membrane protein 2A (LMP2A) in EBV-associated nasopharyngeal carcinoma. J Pathol 2004;203:696-9. [Crossref] [PubMed]
- Meij P, Leen A, Rickinson AB, et al. Identification and prevalence of CD8(+) T-cell responses directed against Epstein-Barr virus-encoded latent membrane protein 1 and latent membrane protein 2. Int J Cancer 2002;99:93-9. [Crossref] [PubMed]
- Rancan C, Schirrmann L, Hüls C, et al. Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells. PLoS Pathog 2015;11:e1004906. [Crossref] [PubMed]
- Brooks L, Yao QY, Rickinson AB, et al. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol 1992;66:2689-97. [PubMed]
- Smith C, Wakisaka N, Crough T, et al. Discerning regulation of cis- and trans-presentation of CD8+ T-cell epitopes by EBV-encoded oncogene LMP-1 through self-aggregation. Blood 2009;113:6148-52. [Crossref] [PubMed]
- Decaussin G, Sbih-Lammali F, de Turenne-Tessier M, et al. Expression of BARF1 gene encoded by Epstein-Barr virus in nasopharyngeal carcinoma biopsies. Cancer Res 2000;60:5584-8. [PubMed]
- Seto E, Yang L, Middeldorp J, et al. Epstein-Barr virus (EBV)-encoded BARF1 gene is expressed in nasopharyngeal carcinoma and EBV-associated gastric carcinoma tissues in the absence of lytic gene expression. J Med Virol 2005;76:82-8. [Crossref] [PubMed]
- Martorelli D, Houali K, Caggiari L, et al. Spontaneous T cell responses to Epstein-Barr virus-encoded BARF1 protein and derived peptides in patients with nasopharyngeal carcinoma: bases for improved immunotherapy. Int J Cancer 2008;123:1100-7. [Crossref] [PubMed]
- Lin X, Gudgeon NH, Hui EP, et al. CD4 and CD8 T cell responses to tumour-associated Epstein-Barr virus antigens in nasopharyngeal carcinoma patients. Cancer Immunol Immunother 2008;57:963-75. [Crossref] [PubMed]
- Li J, Zeng X, Mo H, et al. Functional inactivation of EBV-specific T-lymphocytes in nasopharyngeal carcinoma: implications for tumor immunotherapy. PLoS ONE 2007;2:e1122. [Crossref] [PubMed]
- Lee SP, Chan AT, Cheung ST, et al. CTL control of EBV in nasopharyngeal carcinoma (NPC): EBV-specific CTL responses in the blood and tumors of NPC patients and the antigen-processing function of the tumor cells. J Immunol 2000;165:573-82. [Crossref] [PubMed]
- Fogg MH, Wirth LJ, Posner M, et al. Decreased EBNA-1-specific CD8+ T cells in patients with Epstein-Barr virus-associated nasopharyngeal carcinoma. Proc Natl Acad Sci U S A 2009;106:3318-23. [Crossref] [PubMed]
- Fogg M, Murphy JR, Lorch J, et al. Therapeutic targeting of regulatory T cells enhances tumor-specific CD8+ T cell responses in Epstein-Barr virus associated nasopharyngeal carcinoma. Virology 2013;441:107-13. [Crossref] [PubMed]
- Lau KM, Cheng SH, Lo KW, et al. Increase in circulating Foxp3+CD4+CD25(high) regulatory T cells in nasopharyngeal carcinoma patients. Br J Cancer 2007;96:617-22. [Crossref] [PubMed]
- Schuler PJ, Harasymczuk M, Schilling B, et al. Effects of adjuvant chemoradiotherapy on the frequency and function of regulatory T cells in patients with head and neck cancer. Clin Cancer Res 2013;19:6585-96. [Crossref] [PubMed]
- Khanna R, Busson P, Burrows SR, et al. Molecular characterization of antigen-processing function in nasopharyngeal carcinoma (NPC): evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res 1998;58:310-4. [PubMed]
- Cai MB, Han HQ, Bei JX, et al. Expression of human leukocyte antigen G is associated with prognosis in nasopharyngeal carcinoma. Int J Biol Sci 2012;8:891-900. [Crossref] [PubMed]
- Klibi J, Niki T, Riedel A, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 2009;113:1957-66. [Crossref] [PubMed]
- Gourzones C, Barjon C, Busson P. Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer Biol 2012;22:127-36. [Crossref] [PubMed]
- Yip WK, Abdullah MA, Yusoff SM, et al. Increase in tumour-infiltrating lymphocytes with regulatory T cell immunophenotypes and reduced zeta-chain expression in nasopharyngeal carcinoma patients. Clin Exp Immunol 2009;155:412-22. [Crossref] [PubMed]
- Ben-Haj-Ayed A, Moussa A, Ghedira R, et al. Prognostic value of indoleamine 2,3-dioxygenase activity and expression in nasopharyngeal carcinoma. Immunol Lett 2016;169:23-32. [Crossref] [PubMed]
- Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 2013;19:3462-73. [Crossref] [PubMed]
- Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014;5:12189-202. [Crossref] [PubMed]
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015;14:561-84. [Crossref] [PubMed]
- Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013;5:200ra116.
- Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37.
- Kanakry JA, Ambinder RF. EBV-related lymphomas: new approaches to treatment. Curr Treat Options Oncol 2013;14:224-36. [Crossref] [PubMed]
- Gottschalk S, Ng CY, Perez M, et al. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood 2001;97:835-43. [Crossref] [PubMed]
- Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 2007;110:1123-31. [Crossref] [PubMed]
- Straathof KC, Bollard CM, Popat U, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus--specific T lymphocytes. Blood 2005;105:1898-904. [Crossref] [PubMed]
- Lutzky VP, Crooks P, Morrison L, et al. Cytotoxic T cell adoptive immunotherapy as a treatment for nasopharyngeal carcinoma. Clin Vaccine Immunol 2014;21:256-9. [Crossref] [PubMed]
- Cottingham MG, Carroll MW. Recombinant MVA vaccines: dispelling the myths. Vaccine 2013;31:4247-51. [Crossref] [PubMed]
- Taylor GS, Haigh TA, Gudgeon NH, et al. Dual stimulation of Epstein-Barr Virus (EBV)-specific CD4+- and CD8+-T-cell responses by a chimeric antigen construct: potential therapeutic vaccine for EBV-positive nasopharyngeal carcinoma. J Virol 2004;78:768-78. [Crossref] [PubMed]
- Smith C, Beagley L, Khanna R. Acquisition of polyfunctionality by Epstein-Barr virus-specific CD8+ T cells correlates with increased resistance to galectin-1-mediated suppression. J Virol 2009;83:6192-8. [Crossref] [PubMed]
- Ning RJ, Xu XQ, Chan KH, et al. Long-term carriers generate Epstein-Barr virus (EBV)-specific CD4(+) and CD8(+) polyfunctional T-cell responses which show immunodominance hierarchies of EBV proteins. Immunology 2011;134:161-71. [Crossref] [PubMed]
- Paludan C, Bickham K, Nikiforow S, et al. Epstein-Barr nuclear antigen 1-specific CD4(+) Th1 cells kill Burkitt’s lymphoma cells. J Immunol 2002;169:1593-603. [Crossref] [PubMed]
- Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol 2005;23:720-31. [Crossref] [PubMed]
- Blum KS, Pabst R. Lymphocyte numbers and subsets in the human blood. Do they mirror the situation in all organs? Immunol Lett 2007;108:45-51. [Crossref] [PubMed]
- Parsonage G, Machado LR, Hui JW, et al. CXCR6 and CCR5 localize T lymphocyte subsets in nasopharyngeal carcinoma. Am J Pathol 2012;180:1215-22. [Crossref] [PubMed]
- Teichmann M, Meyer B, Beck A, et al. Expression of the interferon-inducible chemokine IP-10 (CXCL10), a chemokine with proposed anti-neoplastic functions, in Hodgkin lymphoma and nasopharyngeal carcinoma. J Pathol 2005;206:68-75. [Crossref] [PubMed]
- Schijns V, Tartour E, Michalek J, et al. Immune adjuvants as critical guides directing immunity triggered by therapeutic cancer vaccines. Cytotherapy 2014;16:427-39. [Crossref] [PubMed]
- Satti I, Meyer J, Harris SA, et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial. Lancet Infect Dis 2014;14:939-46. [Crossref] [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Hodge JW, Ardiani A, Farsaci B, et al. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol 2012;39:323-39. [Crossref] [PubMed]
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012;12:237-51. [Crossref] [PubMed]
- Adams JL, Smothers J, Srinivasan R, et al. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov 2015;14:603-22. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Foy SP, Sennino B, Dela Cruz T, et al. Poxvirus-Based Active Immunotherapy with PD-1 and LAG-3 Dual Immune Checkpoint Inhibition Overcomes Compensatory Immune Regulation, Yielding Complete Tumor Regression in Mice. PLoS ONE 2016;11:e0150084. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Duraiswamy J, Kaluza KM, Freeman GJ, et al. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013;73:3591-603. [Crossref] [PubMed]
- Goding SR, Wilson KA, Xie Y, et al. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol 2013;190:4899-909. [Crossref] [PubMed]
- Lee AW, Ma BB, Ng WT, et al. Management of Nasopharyngeal Carcinoma: Current Practice and Future Perspective. J Clin Oncol 2015;33:3356-64. [Crossref] [PubMed]
- Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012;13:163-71. [Crossref] [PubMed]
- Hale DF, Clifton GT, Sears AK, et al. Cancer vaccines: should we be targeting patients with less aggressive disease? Expert Rev Vaccines 2012;11:721-31. [Crossref] [PubMed]
- Chua ML, Wee JT, Hui EP, et al. Nasopharyngeal carcinoma. Lancet 2016;387:1012-24. [Crossref] [PubMed]
- Cohen JI, Fauci AS, Varmus H, et al. Epstein-Barr virus: an important vaccine target for cancer prevention. Sci Transl Med 2011;3:107fs7.
- Ogembo JG, Muraswki MR, McGinnes LW, et al. A chimeric EBV gp350/220-based VLP replicates the virion B-cell attachment mechanism and elicits long-lasting neutralizing antibodies in mice. J Transl Med 2015;13:50. [Crossref] [PubMed]
- Cui X, Cao Z, Sen G, et al. A novel tetrameric gp350 1-470 as a potential Epstein-Barr virus vaccine. Vaccine 2013;31:3039-45. [Crossref] [PubMed]
- Kanekiyo M, Bu W, Joyce MG, et al. Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell 2015;162:1090-100. [Crossref] [PubMed]
- Long HM, Leese AM, Chagoury OL, et al. Cytotoxic CD4+ T cell responses to EBV contrast with CD8 responses in breadth of lytic cycle antigen choice and in lytic cycle recognition. J Immunol 2011;187:92-101. [Crossref] [PubMed]
- Adhikary D, Behrends U, Moosmann A, et al. Control of Epstein-Barr virus infection in vitro by T helper cells specific for virion glycoproteins. J Exp Med 2006;203:995-1006. [Crossref] [PubMed]


