Alternatives to whole breast irradiation in early breast cancer
Introduction
Breast cancer is a leading cause of non-skin cancer in women with an estimated 1.2 million new cases each year and 500,000 deaths worldwide with an incidence in China of 18.7 per 100,000 and 5.5 deaths per 100,000 (1,2). The current standard of care is to offer breast conservation therapy (BCT) to patients with early stage disease. BCT includes excision of the tumor with a rim of normal tissue (i.e., lumpectomy) and adjuvant treatments including radiation therapy, most commonly whole breast radiation therapy (WBXRT), to treat residual disease. BCT offers equivalent survival to mastectomy (removal of the whole breast) with similar local recurrence rates (3-6). Furthermore, BCT is less invasive and has favorable cosmetic and psychological outcomes compared to mastectomy (7,8).
However, BCT has limitations. Approximately 24% of BCT patients undergo at least one additional surgery due to inadequate negative margins on the initial lumpectomy (9) [83.2% of these additional surgeries are mastectomies (10)] at a total cost of $234 million/year to the United States (US) healthcare system (11). Additionally, BCT patients undergo 25–30 adjuvant WBXRT sessions over 3–6 weeks, introducing adverse effects, high costs, and inconvenience (12-15). For example, radiation dermatitis, breast shrinkage, pneumonitis/pulmonary fibrosis, edema, secondary solid cancers, and ischemic heart disease are known WBXRT complications and sequelae (16-20). Patients also often endure lengthy travel and significant expenses ($13,000–$47,000) (13,14) to access WBXRT facilities, leading many that may prefer breast conservation to elect mastectomy (14,15). Unfortunately, 36% of women that elect mastectomy are actually eligible for BCT. Additionally, due to the challenges of WBXRT, 14–26% of BCT patients skip WBXRT entirely, and an additional 22% of women do not complete their treatment (21-23). Patients refusing completion radiation led to trials testing whether older patients (>70 years old) with favorable tumors received significant benefit from radiation. While the initial results looked favorable, the most recent follow-up of the Cancer and Leukemia Group B (CALGB) 9343 demonstrated at 10 years that 98% of patients receiving Tamoxifen plus WBXRT were free from local and regional recurrence compared to only 90% of those receiving tamoxifen alone (24). Survival was equivalent. This is tempered, however, with the fact that the Early Breast Cancer Trialist Group who reviewed data from over 42,000 women from 78 randomized studies demonstrated that at 15-year follow-up, for every four recurrences there was an associated increase of one death that could be avoided (6).
These issues have led to the development of several alternatives to WBXRT; unfortunately, these also have inherent limitations. Accelerated partial breast irradiation (APBI) technologies deliver biologically equivalent doses of radiation twice daily for five days to the 1-cm region around the lumpectomy site where up to 90% of recurrences occur (25-27). These technologies include a number of techniques and a plethora of devices developed for delivery of shorter courses of radiation administered only to the pericavitary breast tissue including interstitial catheters(not in use in more modern practice) (28), applicator-based brachytherapy (29), and external-beam XRT (30).
Accelerated partial breast irradiation (APBI)
External-beam XRT
One option for avoiding whole breast irradiation after breast conserving surgery is the use of 3-dimensional conformal external beam radiation therapy (3D-CRT) to deliver noninvasive APBI. This modality may be advantageous to brachytherapy in comparison due to its noninvasive nature, widespread availability of necessary resources, knowledge of final pathology at the time of treatment planning, and potential decrease in seroma formation and infection associated with catheter-based treatment. APBI is most commonly administered in a 38.5-Gy regimen divided into 10 fractions given twice per day for 5 days. Rodríguez et al. reported on the five-year outcomes of 102 patients with early stage breast cancer randomized to receive WBXRT or APBI (31). A median follow-up of 5 years found no local recurrences in either group, and physician assessment reported that >75% of patients had excellent or good cosmesis and remained stable over time (31). An interim analysis of the RAPID (randomized trial of APBI) trial was performed by Peterson and colleagues to evaluate cosmetic outcomes of APBI compared to WBRT (32). The RAPID trial enrolled 1,108 patients; 539 were randomized to 3-D external beam APBI and 569 were randomized to WBRT. Baseline post-treatment nurse assessment for adverse cosmesis was 19% in the APBI arm and 17% in the WBRT arm with 3 year evaluations increased to 29% with adverse cosmetic outcomes for APBI, but remained 17% for WBRT (P<0.001) (32). The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39/Radiation Therapy Oncology Group (RTOG) 0413 trial that randomized 3,000 patients to WBXRT or partial breast irradiation (PBI) has not yet matured. Most of the patients on the non-WBXRT arm have received 3D-CRT (33).
The American Society for Radiation Oncology (ASTRO) released a consensus statement in 2009 that stratified patients into suitable, cautionary, and unsuitable groups in terms of APBI stating that 3D-CRT offers excellent target coverage and dose homogeneity, but may have increased doses to the surrounding uninvolved tissues and inferior conformality. Intensity-modulated radiotherapy (IMRT) and tomotherapy (Tomo-Therapy Incorporated, Madison, WI, USA) for CT guidance may assist in resolving these issues (34). In 2012, an evaluation of the consensus statement recommendations reviewed 2,127 cases of APBI, with 206 of those patients having 3D-CRT. There was no statistically significant difference in the stratified ASTRO groups in terms of ipsilateral breast tumor recurrence (IBTR) (P=0.2). At a median follow-up of 6.7 years, the recurrence rate in the tumor bed was 1.1% in the suitable group, 1.2% in the cautionary group and 1.2% in the unsuitable group (P=0.99), supporting the safety of this method (35). Although 3D-CRT has been readily accepted, there is much more reported experience in catheter-based radiation. Most of that experience is in the form of single-institution experience or registries.
Catheter based radiation therapy (brachytherapy)
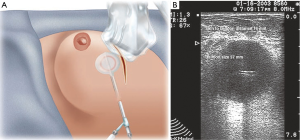
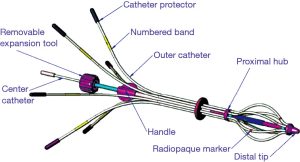
MammoSite™ (Hologic, Marlborough, MA, USA) the first balloon-based catheter was Food and Drug Administration (FDA) approved in 2002. It consists of a single treatment lumen and is available in different sizes (4–5 and 5–6 cm) and shapes (spherical versus elliptical) (Figure 1A). The device delivers 34 Gy in 10 divided doses over a 5-day period. The Mammosite Registry of the American Society of Breast Surgeons has the largest collection of data with this device which demonstrates comparable results to historical controls receiving WBXRT (37). The registry has 1,449 patients with a median follow of 63.1 months as published in 2013. The 5-year actuarial IBTR rate is 3.8% and axillary recurrence rate is 0.6% which is comparable to the rates for whole breast irradiation. The main disadvantages are the minimum distance of skin required from skin to cavity which is about 7 mm and the need for the balloon to approximate the excision cavity (Figure 1B). Cosmetic results are good to excellent in about 90% of the patients. Contura™ (SenoRx, Inc. Aliso Viejo, CA, USA) is a similar balloon catheter that has multiple catheters within the balloon and also comes in similar sizes. The multiple catheters offer a total of 40 dwell positions that allow more precise treatment planning and thus the skin to cavity distance can be narrower (Figure 2). This balloon also has vacuum ports that enable the removal of fluid and air. The main disadvantage of this catheter is its stiffness and thus is more uncomfortable for the patient. Mammosite also introduced a multi-lumen catheter in 2009. The SAVI™ (Cianna Medical, Inc., Aliso Viejo, CA, USA) device which also has multicatheters (6, 8, or 10) has the advantage that it can be custom fitted to the lumpectomy cavity. Each device consists of a central lumen and then multiple outer lumens (Figure 3), allowing better tailoring of radiation to the cavity. Like the Contura, the SAVI is very stiff. ClearPath™ (Renata Medical, Irvine, CA, USA) makes a similar device with multiple catheters and is advantageous in that it has a lower profile laying flush with the skin, minimizing external exposure of the catheter, and is better tolerated by the patient (Figure 4). Xoft, Inc. (Sunnyvale, CA, USA) has developed the Axxent® electronic brachytherapy system that is an iridium seed-based single catheter balloon impregnated with barium that emits low energy and can be turned on and off such that it can be used in the office setting. The applicator design includes drainage lumens which allow better approximation of the catheter to the excision cavity (Figure 5). There is a separate port for insertion of the X-ray source and the balloon is radiolucent to improve visibility on breast radiographs and CT images and therefore unlike the other balloons does not require contrast. The inter-societal Electronic Xoft Intersocietal Brachytherapy Trial (EXIBT) registry recruited 400 patients and at one year follow-up demonstrated the safety and efficacy of Xoft as an adjuvant radiation therapy for early-stage breast cancer (38).



In general multi-lumen applicators offer the advantages of more flexibility in planning and the ability to overcome dose constraints better than single lumen applicators. The other advantage of brachytherapy in general is that the radiation therapy can be completed prior to systemic therapy although the initiation of chemotherapy should be delayed for ~three weeks to avoid radiation recall and skin wall necrosis and damage. Disadvantages with multi-lumen applicators (excluding SAVI which can be locked into place) are that they can rotate leading to incorrect dose delivery; increased seromas are seen more with intraoperatively versus post-operative placed balloon applicators; skin to applicator surface can decrease over treatment course leading to the need to scan the applicator daily to assure tissue to applicator approximation and as with WBXRT high dose regions can be significantly large and lead to fat necrosis and poor cosmetic result.
Recent ASTRO guidance states that APBI is suitable for a subset of BCT patients with tumors <3 cm when margin width exceeds 2 mm, but the supporting data have been controversial (34,39-41). Most significantly, APBI has been associated with a 4-fold higher rate of patients developing a palpable mass at the lumpectomy site and a 42% incidence of telangiectasia and/or other side effects (42).
Intraoperative radiation therapy
A recent 5-year study of another partial breast treatment method, intra-operative radiation therapy (IORT), demonstrated that local therapy can provide effective long-term local control with equivalent recurrence rates to WBRT (43). IORT is more wide spread in Europe than in the US or China. IORT delivers a single large fraction over 2 minutes directly into the wound for increased dose delivery and decreased normal tissue exposure. IORT is more expensive because it requires radiation delivery equipment not available at most hospitals. However, as the equipment becomes more available and the costs go down, more surgeons and radiation oncologists will offer it as a safe alternative to WBXRT (44).
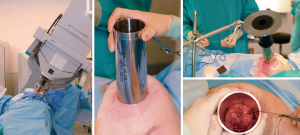
The Mobetron IORT device is a self-shielded, magnetron-driven, X-band linear accelerator specifically designed for intraoperative use (45). The breast tissue is mobilized over a lead/aluminum shield placed posteriorly to protect the underlying chest wall and viscera. The applicators as shown in Figure 6 are of a different design and come in sizes that range from 3 to 10 cm in diameter. The applicator is placed inside the incision and the skin dissected to some extent to minimize skin dosage. Then the applicator and patient are placed under the Mobetron and laser alignment is used to position the gantry. The operating room team then leaves the operating room while the radiation is delivered. Then the devices are removed and the wound closed. Philippson and colleagues recruited 200 patients to a phase II trial who had breast cancer less than or equal to 2 cm and no lymph node involvement, lymphovascular invasion or extensive ductal carcinoma in situ (DCIS) received 21 Gy intraoperatively (47). At a median follow-up of 23 months the local regional recurrence was 0.5%, acute toxicity was 5.4%, and the late toxicity was 17.1%. Cosmetic outcome was good or very good in 92.5% of patients. Some of the major disadvantages to Mobetron-based IORT are the lack of knowledge as to the margin status intraoperatively and the cost of the system at well over a million dollars in the US in addition to the need for dedicated space and multiple disciplines to be present in the operating room requiring coordination of services.
The ELIOT trial also uses intraoperative electrons (IOERT) (48,49). After tumor excision, the breast tissue is mobilized over a lead/aluminum shield to protect the ribs and underlying viscera. The peri-cavitary tissue is then approximated via purse string around a collimator (4–9 cm). The linear accelerator is then used to deliver 21 Gy to the tumor bed. The ELIOT trial compared WBXRT to IOERT in a trial in which 1,205 patients presented with tumors 2.5 cm or smaller. In the intent-to-treat analysis the 5-year IBTR was 4.7% for ELIOT versus 0.5% for WBXRT. For low risk women the 5-year IBTR was 1.7%. For patients with one or more high risk features (tumor size, receptor status, nodal positivity and grade) the rate of 5-year IBTR was significantly higher at 11.3%. ELIOT did report less skin and pulmonary damage but a higher rate of regional failure (1.0% vs. 0.3%, P=0.03). There was no difference in terms of pain, retraction or fibrosis. Overall survival was the same between the two arms.
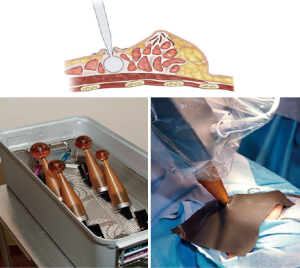
Zeiss (Carl Zeiss Meditec, Jena, Germany) has developed a low energy electronic radiation source (IntraBeam™) for intraoperative PBI that is much more similar than Mobetron to the balloon catheters (Figure 7). The applicators are solid and rounded and come in different sizes to place into the wound. After the lumpectomy is performed a purse string is placed and ultrasound is used to confirm that the applicator is juxtaposed to the tissue of the excision cavity. Skin edges are sutured out of the field to avoid skin damage. A 20-Gy one time dose is delivered at the surface of the applicator such that a dose of 5 Gy is delivered at a depth of 1cm from the cavity. Shielding is required to reduce radiation scatter. The radiation oncologist must calculate the time of treatment based on the size of the applicator. The IntraBeam shares some of the same disadvantages as the IOERT devices but is much less expensive and has long-term data demonstrating non-inferiority to WBXRT as well as a two percent survival advantage for cardiac events thought attributable to radiation (43). Silverstein and colleagues recently published a critical analysis and comparison of the two studies (51).
Intraoperative radiofrequency ablation (RFA)
The successful demonstration of local therapy alternatives to WBXRT opens new opportunities for BCT patients to achieve positive outcomes without the cost, inconvenience, and morbidities associated with WBXRT or APBI. Our group hypothesized that radiofrequency ablation (RFA) of breast tissue around the post-lumpectomy cavity would reduce re-excision rates for patients with close or positive margins, as well as potentially provide local tumor control without radiation (52,53). Similar to other methods of local control, RFA of the margin avoids damage to nearby organs including the heart and the lung that can occur with WBXRT. RFA is a method of inducing thermal tissue necrosis by using an applied sinusoidal voltage, oscillating between 400–800 kHz between electrodes and a dispersive grounding pad (in monopolar RFA) to generate ionic heating, and is currently utilized for the treatment of liver, lung, kidney, and bone tumors (54,55).
We initially investigated our RFA by performing lumpectomies in donor mastectomy samples, followed with RFA of the cavity to temperatures of 100 °C. The procedure involves purse-string suturing the cavity to a ~1.0 cm volume, then the electrode tines of the radiofrequency probe are deployed into the cavity walls to achieve a circumferential ablation zone averaging 5–10 mm in depth from the cavity wall (53). This preliminary investigation led to a five-year 100-patient pilot trial including 22 patients with close or positive margins. Of these, 15 did not require re-excision per protocol. Upon follow-up, two of 68 patients who received a wide excision followed by RFA alone had a true in-breast recurrence (i.e., two elsewhere recurrences) and three had needle biopsy skin tract recurrences that were not classified as in-breast recurrence, but there were no tumor bed recurrences. The 5-year disease free survival rate was 88% (53). The post-operative complication rate was 6% (wound dehiscence, hematomas, or infection), and the cosmetic outcome on four-point scale was graded as excellent or good in 92% of patients. Furthermore, we have just completed a 250 patient multi-center trial. Results will be reported as the trial matures (56).
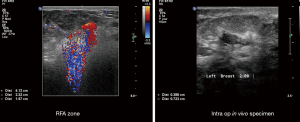
One of the issues that had limited adoption of RFA was the initial inability to determine the progression and size of the ablation zone. Doppler ultrasound is currently utilized to observe nitrogen off-gassing from tissue motion (57) in the tissue during ablation and therefore gives an estimation of the ablated zone (Figure 8). This is an essential component to the success of breast tumor ablation both primarily and secondarily that is excision followed by ablation. Accurate imaging of the ablated region is necessary for evaluating the clinical endpoint of these procedures which is achieving a 1cm region of necrosis around a tumor to avoid re-excision in case of close or focally positive margins as well as to mirror the equivalent area of radiation given during brachytherapy.
Experimental percutaneous ablation techniques
There is a desire to eventually be able to percutaneously ablate tumors. Promising yet experimental percutaneous techniques include RFA, cryoablation, laser, microwave therapy, focused microwave thermotherapy, percutaneous microwave coagulation, high-intensity focused ultrasound ablation (HIFU). Most trials have less than 100% ablation of whole breast tumors and especially larger lesions and those that contain DCIS (58).
RFA of breast cancer tumors without surgical removal has also been investigated (59-61). However, percutaneous RFA of primary breast tumors has met resistance and has found limited adoption by oncologists, breast surgeons, and patients because ablation of the primary tumor does not allow for complete histological examination of the tumor and its margin, essentially eliminating tissue banking for molecular profiling and other future therapeutic and prevention options.
Klimberg et al. used percutaneous excision of lesions less than or equal to 1.5 cm by vacuum-assisted device followed by percutaneous RFA versus laser as an alternative treatment to open lumpectomy. Twenty-one patients were enrolled onto a pilot trial with fifteen receiving RFA after percutaneous excision. In this manner 100 percent ablation and negative margins were achieved. However, the laser arm was stopped secondary to unreliable ablation zones (62). Further follow-up studies are needed to determine if this is a reliable option for small favorable breast cancers.
Summary
There is no doubt that radiation therapy reduces the risk of recurrence but only in the peri-cavitary bed. Thus it makes sense that PBI or treatment through whatever method should be effective in maintaining local control with decreased toxicity. Thus the treatment of breast cancer by BCT continues to evolve as do the methods of delivery of radiation and other methods to the tumor bed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Society AC. What are the key statistics about breast cancer? 2014; Available online: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics
- Liu JJ, Zhang S, Hao X, et al. Breast-conserving therapy versus modified radical mastectomy: socioeconomic status determines who receives what--results from case-control study in Tianjin, China. Cancer Epidemiol 2012;36:89-93. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment ofinvasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol 2005;28:289-94. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Munshi A, Kakkar S, Bhutani R, et al. Factors influencing cosmetic outcome in breast conservation. Clin Oncol (R Coll Radiol) 2009;21:285-93. [Crossref] [PubMed]
- Al-Ghazal SK, Fallowfield L, Blamey RW. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer 2000;36:1938-43. [Crossref] [PubMed]
- Wilke LG, Czechura T, Wang C, et al. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004-2010. JAMA Surg 2014;149:1296-305. [Crossref] [PubMed]
- Landercasper J, Whitacre E, Degnim AC, et al. Reasons for re-excision after lumpectomy for breast cancer: insight from the American Society of Breast Surgeons Mastery(SM) database. Ann Surg Oncol 2014;21:3185-91. [Crossref] [PubMed]
- Osborn JB, Keeney GL, Jakub JW, et al. Cost-effectiveness analysis of routine frozen-section analysis of breast margins compared with reoperation for positive margins. Ann Surg Oncol 2011;18:3204-9. [Crossref] [PubMed]
- Breast Cancer Treatment (PDQ). 2014; Available online: http://www.cancer.gov/cancertopics/pdq/treatment/breast/Patient/page1
- Greenup RA, Camp MS, Taghian AG, et al. Cost comparison of radiation treatment options after lumpectomy for breast cancer. Ann Surg Oncol 2012;19:3275-81. [Crossref] [PubMed]
- Goyal S, Chandwani S, Haffty BG, et al. Effect of travel distance and time to radiotherapy on likelihood of receiving mastectomy. Ann Surg Oncol 2015;22:1095-101. [Crossref] [PubMed]
- Athas WF, Adams-Cameron M, Hunt WC, et al. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst 2000;92:269-71. [Crossref] [PubMed]
- Berrington de Gonzalez A, Curtis RE, Gilbert E, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer 2010;102:220-6. [Crossref] [PubMed]
- Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol 2008;26:1239-46. [Crossref] [PubMed]
- Lind PA, Marks LB, Hardenbergh PH, et al. Technical factors associated with radiation pneumonitis after local +/- regional radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys 2002;52:137-43. [Crossref] [PubMed]
- Meric F, Buchholz TA, Mirza NQ, et al. Long-term complications associated with breast-conservation surgery and radiotherapy. Ann Surg Oncol 2002;9:543-9. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 2015;150:9-16. [Crossref] [PubMed]
- Pan IW, Smith BD, Shih YC. PCN126 Radiation After Breast-Conserving Surgery: Compliance of Clinical Guideline Among Young Women With Employment-Based Insurance in the United States. Value in Health 2012;15:A230-A231. [Crossref]
- Ramsey SD, Zeliadt SB, Richardson LC, et al. Discontinuation of radiation treatment among medicaid-enrolled women with local and regional stage breast cancer. Breast J 2010;16:20-7. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 2013;31:2382-7. [Crossref] [PubMed]
- Fisher ER, Anderson S, Redmond C, et al. Ipsilateral breast tumor recurrence and survival following lumpectomy and irradiation: pathological findings from NSABP protocol B-06. Semin Surg Oncol 1992;8:161-6. [PubMed]
- Fisher ER, Anderson S, Tan-Chiu E, et al. Fifteen-year prognostic discriminants for invasive breast carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol-06. Cancer 2001;91:1679-87. [Crossref] [PubMed]
- Vaidya JS, Tobias JS, Baum M, et al. Intraoperative radiotherapy for breast cancer. Lancet Oncol 2004;5:165-73. [Crossref] [PubMed]
- King TA, Bolton JS, Kuske RR, et al. Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T(is,1,2) breast cancer. Am J Surg 2000;180:299-304. [Crossref] [PubMed]
- Benitez PR, Keisch ME, Vicini F, et al. Five-year results: the initial clinical trial of MammoSite balloon brachytherapy for partial breast irradiation in early-stage breast cancer. Am J Surg 2007;194:456-62. [Crossref] [PubMed]
- Chen PY, Wallace M, Mitchell C, et al. Four-year efficacy, cosmesis, and toxicity using three-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys 2010;76:991-7. [Crossref] [PubMed]
- Rodríguez N, Sanz X, Dengra J, et al. Five-year outcomes, cosmesis, and toxicity with 3-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys 2013;87:1051-7. [Crossref] [PubMed]
- Peterson D, Truong PT, Parpia S, et al. Predictors of adverse cosmetic outcome in the RAPID trial: an exploratory analysis. Int J Radiat Oncol Biol Phys 2015;91:968-76. [Crossref] [PubMed]
- Radiation Therapy Oncology Group. NSABP Protocol B-39/RTOG Protocol 0413: a randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with stage 0, I, or II breast cancer. Clin Adv Hematol Oncol 2004;4:719-21.
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987-1001. [Crossref] [PubMed]
- Wilkinson JB, Beitsch PD, Shah C, et al. Evaluation of current consensus statement recommendations for accelerated partial breast irradiation: a pooled analysis of William Beaumont Hospital and American Society of Breast Surgeon MammoSite Registry Trial Data. Int J Radiat Oncol Biol Phys 2013;85:1179-85. [Crossref] [PubMed]
- Beitsch P. Mammosite balloon catheter placement and other brachytherapy delivery devices. In: Klimberg VS, editor. Atlas of Breast Surgical Techniques. Philadelphia: Saunders Elsevier, 2010:389. Series Editors: Townsend C, Evers M.
- Shah C, Badiyan S, Ben Wilkinson J, et al. Treatment efficacy with accelerated partial breast irradiation (APBI): final analysis of the American Society of Breast Surgeons MammoSite(®) breast brachytherapy registry trial. Ann Surg Oncol 2013;20:3279-85. [Crossref] [PubMed]
- Beitsch PD, Patel RR, Lorenzetti JD, et al. Post-surgical treatment of early-stage breast cancer with electronic brachytherapy: an intersociety, multicenter brachytherapy trial. Onco Targets Ther 2010;3:211-8. [Crossref] [PubMed]
- Patel PS, Nori D, Monni S, et al. Outcomes of Patients in ASTRO's Cautionary Group Treated with Accelerated Partial Breast Irradiation (APBI). Int J Radiat Oncol Biol Phys 2010;78:S220. [Crossref]
- Polgár C, Major T, Fodor J, et al. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother Oncol 2010;94:274-9. [Crossref] [PubMed]
- Vicini FA, Antonucci JV, Wallace M, et al. Long-term efficacy and patterns of failure after accelerated partial breast irradiation: a molecular assay-based clonality evaluation. Int J Radiat Oncol Biol Phys 2007;68:341-6. [Crossref] [PubMed]
- Rosenkranz KM, Tsui E, McCabe EB, et al. Increased rates of long-term complications after MammoSite brachytherapy compared with whole breast radiation therapy. J Am Coll Surg 2013;217:497-502. [Crossref] [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [Crossref] [PubMed]
- Ollila DW, Klauber-DeMore N, Tesche LJ, et al. Feasibility of breast preserving therapy with single fraction in situ radiotherapy delivered intraoperatively. Ann Surg Oncol 2007;14:660-9. [Crossref] [PubMed]
- Stitzenberg KB, Klauber-Demore N, Chang XS, et al. In vivo intraoperative radiotherapy: a novel approach to radiotherapy for early stage breast cancer. Ann Surg Oncol 2007;14:1515-6. [Crossref] [PubMed]
- Klauber-DeMore N, Sartor CI, Ollila DW. In vivo Intraoperative radiation therapy for breast cancer. In: Klimberg VS, editor. Atlas of Breast Surgical Techniques. Philadelphia: Saunders Elsevier, 2010. Series Editors: Townsend C, Evers M.
- Philippson C, Simon S, Vandekerkhove C, et al. Early invasive cancer and partial intraoperative electron radiation therapy of the breast: experience of the jules bordet institute. Int J Breast Cancer 2014;2014:627352.
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [Crossref] [PubMed]
- Silverstein MJ, Fastner G, Maluta S, et al. Intraoperative radiation therapy: a critical analysis of the ELIOT and TARGIT trials. Part 1--ELIOT. Ann Surg Oncol 2014;21:3787-92. [Crossref] [PubMed]
- McCready DR, Henderson MA. Targeted Intraoperative Radiation Therapy (TARGIT). In: Klimberg VS, editor. Atlas of Breast Surgical Techniques. Philadelphia: Saunders Elsevier, 2010. Series Editors: Townsend C, Evers M.
- Silverstein MJ, Fastner G, Maluta S, et al. Intraoperative radiation therapy: a critical analysis of the ELIOT and TARGIT trials. Part 2--TARGIT. Ann Surg Oncol 2014;21:3793-9. [Crossref] [PubMed]
- Klimberg VS, Kepple J, Shafirstein G, et al. eRFA: excision followed by RFA-a new technique to improve local control in breast cancer. Ann Surg Oncol 2006;13:1422-33. [Crossref] [PubMed]
- Klimberg VS, Ochoa D, Henry-Tillman R, et al. Long-term results of phase II ablation after breast lumpectomy added to extend intraoperative margins (ABLATE l) trial. J Am Coll Surg 2014;218:741-9. [Crossref] [PubMed]
- Ekstrand V, Wiksell H, Schultz I, et al. Influence of electrical and thermal properties on RF ablation of breast cancer: is the tumour preferentially heated? Biomed Eng Online 2005;4:41. [Crossref] [PubMed]
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009;38:135-43. [Crossref] [PubMed]
- Klimberg VS, Korourian S. ABLATE Registry: Radiofrequency Ablation after Breast Lumpectomy (eRFA) Added to Extend Intraoperative Margins in the Treatment of Breast Cancer 2010; Available online: https://www.uamshealth.com/upload/docs/breastteam/2011/ABLATE%20Registry%20Protocol%20vJan52010.doc
- Nahirnyak VM, Moros EG, Novák P, et al. Doppler signals observed during high temperature thermal ablation are the result of boiling. Int J Hyperthermia 2010;26:586-93. [Crossref] [PubMed]
- Fornage BD, Hwang RF. Current status of imaging-guided percutaneous ablation of breast cancer. AJR Am J Roentgenol 2014;203:442-8. [Crossref] [PubMed]
- Fornage BD, Sneige N, Ross MI, et al. Small (< or = 2-cm) breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology 2004;231:215-24. [Crossref] [PubMed]
- Singletary SE, Fornage BD, Sneige N, et al. Radiofrequency ablation of early-stage invasive breast tumors: an overview. Cancer J 2002;8:177-80. [Crossref] [PubMed]
- Noguchi M, Earashi M, Fujii H, et al. Radiofrequency ablation of small breast cancer followed by surgical resection. J Surg Oncol 2006;93:120-8. [Crossref] [PubMed]
- Klimberg VS, Boneti C, Adkins LL, et al. Feasibility of percutaneous excision followed by ablation for local control in breast cancer. Ann Surg Oncol 2011;18:3079-87. [Crossref] [PubMed]


