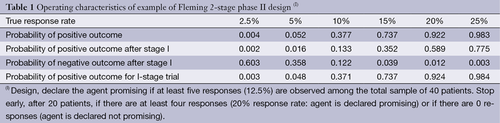
Historically, phase II trials in oncology generally had a single-arm design, constructed to distinguish between a tumor response rate felt to indicate a lack of promise (often 5%) and a rate that would indicate potential benefit (often 20%), with a one-sided type I error rate of 5% to 10% and a type II error rate of 10% to 20%. We have reviewed the history and evolution of the phase II trial over the past 50 years, in particular, in oncology trials.