Percutaneous palliative care interventions in the cancer patient
Introduction
Cancer is the second leading cause of death worldwide, accounting for 17.08% (9.6 million) of all deaths in 2017; in developed countries, this proportion is even higher, closely trailing behind cardiovascular disease (1). While the overall age-standardized death rate of cancers is on a decreasing trend, the absolute number of cancer deaths has increased from 5.7 to 9.6 million since 1990 to 2017 (68% increase) along with increasing population size and life expectancy (1). Together with the progressively lengthening of overall survival, the role of cancer palliative care is ever increasing. Complications resulting from cancer or its treatment are a significant determinant of the quality of life of cancer patients. There is a wide array of percutaneous procedures offered by the interventionist that can alleviate such complications, most of which are minimally invasive and can be performed under local anesthesia or sedation.
Broadly, such procedures can be grouped into several categories including:
- Drainage of collections;
- Decompression and relief of obstruction;
- Pain reduction;
- Gastrointestinal access.
The purpose of this article is to describe the basic concepts of minimally invasive techniques applied as palliative care therapies in the cancer patients. Controversies concerning techniques and products and the need for patient-centered tailored approaches will be discussed.
Drainage of fluid collections
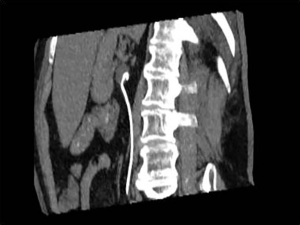
Pleural effusion and ascites are very common in patients with advanced cancer (2-4). These collections are commonly due to pleural or peritoneal metastases as well as indirect causes (3,5). If left untreated, they inflict considerable morbidity and early mortality. Percutaneous temporary drainage of pleural or peritoneal fluid is simple, effective and safe but re-accumulation of fluid is very common as the underlying cause is not corrected. Percutaneous drainage of pleural or peritoneal fluid is simple with minimal risks and could be performed under local anesthesia at the bedside (4,6).
Ultrasound guidance is used in most instances; ultrasound has high sensitivity in detecting even small amounts of pleural effusion or ascites (Figure 1) (6,7). In the case of free pleural fluid, the costophrenic sulcus can be targeted for rapid assessment. Sensitivity can be increased by positioning the torso of the patient in a more upright posture so that fluid gravitates to the costophrenic sulcus. In the case of ascites, the paracolic gutters and pelvic area can be targeted for quick assessment. Similarly, positioning in decubitus or semirecumbent postures can help shift fluid towards the dependent parts of the peritoneal cavity for easier detection and puncture. In the case where fluid is loculated in the pleural or peritoneal space, more thorough scanning is needed to interrogate different parts of the cavities.

Although in patients with symptomatic pleural effusion or ascites, the amount of fluid present is usually large and readily detectable, small amounts of infected pleural or peritoneal fluid can be a cause for significant sepsis especially in the immunocompromised patient. In experienced hands, even tiny amounts of fluid can be safely drained or aspirated, providing material for culture.
Non-tunneled pigtail catheters are the most commonly used type of catheters for temporary pleural and peritoneal drainage (8,9). They are commonly inserted with a direct (one-step approach) approach although the Seldinger technique can also be applied with equally safe results. Serious complications arising from pleural or peritoneal fluid drainage are generally rare, but include infection (the risk increases with the duration of catheter dwelling), bleeding and injury to adjacent organs such as the lungs leading to pneumothorax, the bowels leading to perforation and peritonitis as well as the liver and spleen which could result in hemorrhage (10). The intercostal and the inferior epigastric vessels can rarely be injured leading to significant hemorrhage. Malignant seeding along the catheter track can also occur and is likely more common than reported in the literature. Other complications related to aftercare include blockage and accidental dislodgement of the catheter. Rapid drainage of pleural effusion can precipitate pulmonary edema and large volume drainage of ascites can lead to paracentesis induced circulatory dysfunction (11).
Other types of drainage devices have been developed to facilitate longer term treatment and help patients achieve ambulatory or self-care and minimize hospital stay. These include tunneled catheters, catheters with implantable ports and shunts. Tunneled catheters are designed for a cuffed segment to lie within the subcutaneous layer so that fibrosis with the surrounding tissue occurs. This acts as a barrier to infection from the skin entry as well as protects against dislodgement. Consequently, such catheters can be left in-site for a much longer period of time. Tunneled pleural catheters have emerged as a preferred treatment option for recurrent malignant pleural effusion with trapped lung, endorsed by leading guidelines (8,9). Limited studies have shown that tunneled pleural catheter confers similar control of dyspnea to pleurodesis with shorter hospital stay (12,13). In a meta-analysis, autopleurodesis occurred in 45.6% of patients after an average time of 52 days after tunneled pleural catheter insertion (14).
Pleuroperitoneal shunting has been used in the management of malignant pleural effusion but has not gained popularity; there is insufficient evidence to support their routine use in cancer palliative care (15,16). For malignant ascites, shunting in the form of peritoneovenous shunt has been used however there are limited studies showing efficacy in improving patient quality of life (17-19). In a review in 2011, such shunts provided effective palliation in 75.3% of patients with a complication rate of 38% which included occlusion (24%) and disseminated intravascular coagulation (9%), (17). Pleural and peritoneal drainage systems with implantable port have also been studied with limited studies showing effectiveness in palliation of malignant pleural effusion and ascites, however they have not gained popularity (20,21).
Pleurodesis is the more definitive treatment for recurrent pleural effusion but is suitable only if the lungs can be re-expanded (6). There is no analogous treatment for malignant ascites as while fibrosis in the peritoneal could close up the potential space and might confer similar prevention of re-accumulation of ascites, adhesions on the bowels could lead to bowel obstruction and is not desirable.
Abscesses can occur as a result of tumor due to various reasons such bowel invasion, necrosis or hemorrhage with secondary infection, biliary obstruction and cholangitis, portovenous suppuration and post treatment complications such as from trans-arterial chemoembolization (TACE) and/or ablation; these are more common in the abdomen and pelvis (22). Most of the time, these collections can be drained percutaneously by either CT or ultrasound guidance under local anesthesia.
Decompression and relief of obstruction
Various types of obstruction occur in cancer patients. These include among others biliary, urinary and intestinal obstruction.
Malignant biliary obstruction
Malignant biliary obstruction is common in gastrointestinal and hepatobiliary malignancies as well as in liver metastases; if left untreated, liver function impairment or even infection may lead to early demise of the patient. Endoscopic retrograde cholangio-pancreatography (ERCP) and stenting could act as the first-line treatment for malignant biliary obstruction but is not always possible or successful and percutaneous trans-hepatic biliary drainage can be proposed (23). The biliary ducts can be punctured under direct visualization with ultrasound guidance or by using a trial and error method under fluoroscopy guidance. Once a suitable biliary duct is punctured and confirmed by contrast injection, a wire can be fed into the biliary system followed by dilatation and exchange to a larger wire and catheter using the Seldinger technique. When the catheter is placed upstream of the site of obstruction and bile is drained externally, it is called an external drainage. When the catheter is placed across the site of obstruction such that bile can flow across the catheter pass the site of obstruction back into the small bowels, it is called and internal-external drainage. The problem with external drainages is that the patient can lose large amounts of fluid and electrolytes as well as the important functions bile plays in digestion and absorption of nutrients. An external fluid collection bag that the patient has to carry also affects the quality of life of patients. With internal-external drainages, these problems are eliminated. Internal-external drainage however facilitates communication between the bowels and the biliary system in both directions and as a result introduces bowel flora into the biliary system, predisposing to subsequent cholangitis.
Similar to ERCP, dilatation of stricture and stenting can be performed via the percutaneous trans-hepatic biliary drainage track. Larger caliber metallic stents can be inserted via the percutaneous than the endoscopic route. The success rate of percutaneous trans-hepatic biliary drainage is heavily dependent on the skill of the operator and is higher when the biliary ducts are more dilated; the technique should be performed in centers with appropriate experience and after the optimization of all of the risk factors whilst appropriate patient selection and decrease of risk factors is required to limit high periprocedural morbidity and mortality below 10% (24). In cases where the intrahepatic ducts cannot be accessed and that the site of obstruction is below the cystic duct, percutaneous insertion of drainage catheter into the gallbladder is a viable alternative for biliary drainage provided the cystic duct is patent. Complications of percutaneous trans-hepatic biliary drainage can be puncture/manipulation related including bleeding, infection, injury to vessels, lung base, bowels, bile leak, acute flare of sepsis as well as aftercare related including catheter dislodgement or obstruction, fluid and electrolyte derangement.
Urinary obstruction
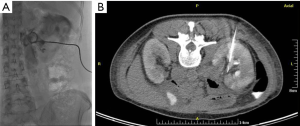
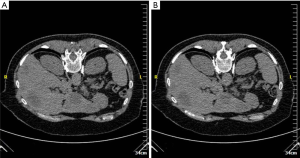
Urinary obstruction is not uncommon in pelvic malignancies such as bladder cancer, gynecological cancers, colorectal cancers as well as retroperitoneal nodal metastases from other primaries with the distal and mid portions of the ureters being the most commonly involved sites (25). Cystoscopic ureteral stenting could provide rapid upper tract urinary decompression but may not always be available or successful. Percutaneous nephrostomy is a simple and safe alternative to effect rapid upper urinary tract decompression (Figure 2). Typically, ultrasound and fluoroscopy are used. Patient is required to be placed into prone position or failing that, in contralateral decubitus position. Ultrasound-guided puncture of the lower pole calyx can then be performed under local anesthesia. Contrast is then injected through the needle to outline the pelvicalyceal system and confirm correct needle position. A wire is then fed into the pelvicalyceal system and a suitable catheter and be exchanged into place using the Seldinger technique. In cases where there is overt hydronephrosis, a catheter can be inserted using a one-step technique. Ureteral stenting can also be performed via a percutaneous nephrostomy tract (Figure 3). Complications of percutaneous nephrostomy include hemorrhage, infection, acute flare of existing urosepsis, injury to adjacent bowels, urine leakage, perforation of the collecting system with urinoma formation, catheter dislodgement and catheter blockage (25).
Small bowel obstruction
Malignant small bowel obstruction is common in patients with advanced malignancies involving the abdomen and pelvis and considerable impacts quality of life (26). When bypass surgery and stenting are deemed non-viable or have failed, percutaneous small bowel decompression may have a role. Jiang et al. reported that percutaneous needle decompression of the small bowels under ultrasound guidance is a safe and effective palliative treatment for small bowel obstruction of malignant substrate; when combined with nasogastric decompression and transarterial chemotherapy, the authors reported a 1-month partial response rate of 94.2% indicating resolution of obstructive symptoms (27). Kim reported percutaneous jejunostomy for small bowel decompression along with self-expandable stenting via the tract in 21 patients with significant improvement in median food intake capacity score, however, dedicated devices should be developed to reduce frequent procedure-related complications (28).
Relief of pain
Cancer pain is common in all phases of the disease and can arise from direct tumor invasion of a pain sensitive structure, complications from tumor and insults from treatments; more than half (56.0–82.3%) of cancer patients have inadequate pain management (29). Percutaneous neurolysis have been shown to be effective in the palliation of intractable pain in cancer patients, particularly for visceral pain mediated by sympathetic axis whilst there are limited data for somatic pain in this group of patients. Various neural targets including the stellate ganglion, thoracic plexus, celiac plexus-splanchnic nerves, lumbar plexus, superior hypogastric plexus and impar ganglion can be successfully destroyed through a percutaneous approach. The most commonly utilized criteria for patient selection are advanced, progressive cancer and a life expectancy of 6–12 months (29).
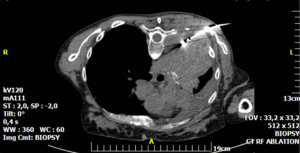
Neurolysis can be affected by chemical (phenol or alcohol) or thermal (radiofrequency, microwave or cryoablation) means (29). Ethanol acts through immediate endoneural lipoprotein and mucoprotein precipitation which causes cholesterol, phospholipid, and cerebroside extraction from the neurilemma; phenol, on the other hand diffuses into axonal and perineural blood vessels causing denaturation of proteins (30-32). In thermal ablation, the probe is placed close to the target nerve tissue and tissue destruction is mediated by heat (radiofrequency or microwave) of up to 60–90 °C or extreme cold (cryoablation) (Figure 4) (29). When a tumor engulfs the neural target(s), these methods effect tumor ablation resulting in additional pain alleviation (29).
Percutaneous neurolysis is usually performed under local anesthesia and conscious sedation. Both ultrasound and CT guidance have been employed for needle placement depending on target location. For celiac plexus neurolysis, endoscopic ultrasound guidance is an alternative approach (33). General complications common to all neurolytic procedures include dysesthesia, neuritis or neuroma formation, hypotension, failure to relieve pain and early return of pain [secondary to central nervous system (CNS) plasticity, axonal regrowth or tumor progression] (29). In the event of an early return of pain, neurolysis may be repeated. Post-neurolysis hypotension is common in celiac, lumbar and superior hypogastric plexus neurolysis and post-procedural bed-rest is required. There are other complications that are location specific. For example, stellate ganglion neurolysis risks tracheal and esophageal perforation, pneumothorax and bleeding or vocal cord palsy leading to airway compromise.
Percutaneous tumor ablation and cementation for bone metastasis
In cancer patients suffering from pain radiotherapy achieves average overall pain responses ranging from 30–60% (34). On the other hand, ablation by means of necrotizing tumor-periosteum interface, by decompression of tumor volume, inhibition of osteoclast activity and decrease in the nerve-stimulating cytokines released by the tumor constitutes an effective means for pain reduction in patients with osseous bone metastases (Figure 5) (35,36). Available techniques include thermal ablation (laser, radiofrequency, microwave ablation, cryoablation, coblation), irreversible electroporation and MR-guided high intensity focused ultrasound (HIFU); all the aforementioned techniques can be proposed as palliative therapy for pain reduction, tumor decompression and debulking (34).
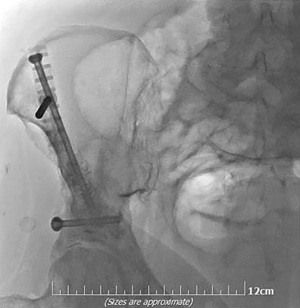
Mechanical pain caused by osseous instability due to bone destruction by metastatic disease can be treated by cement injection either solely performed (percutaneous cementoplasty) or in combination to metalic hardware (34,37-40). The latter combination seems ideal in weight-bearing areas and long bones due to inadequate bone consolidation and the directions’ variety of the applied forces (Figure 6) (37,40).
Conclusions
This article provides a brief overview of various percutaneous interventions that may be useful in the palliative care of cancer patients. The decision-making process involved in prescribing many of these interventions require much more in-depth knowledge in the specific areas and should be tailored to the individual patient. Availability of interventional expertise and local practice have a large bearing on the choice of treatment and good communication between clinicians and interventionists is indispensable to optimal palliative care for the cancer patient.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Global Burden of Disease Collaborative Network. Global burden of disease study 2017 (GBD 2017) results. Seattle: Institute for Health Metrics and Evaluation (IHME), 2018.
- Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 2008;83:235-50. [Crossref] [PubMed]
- Ferreiro L, Suárez-Antelo J, Valdés L. Pleural procedures in the management of malignant effusions. Ann Thorac Med 2017;12:3-10. [Crossref] [PubMed]
- Runyon BA. Care of patients with ascites. N Engl J Med 1994;330:337-42. [Crossref] [PubMed]
- Runyon BA, Hoefs JC, Morgan TR. Ascitic fluid analysis in malignancy-related ascites. Hepatology 1988;8:1104-9. [Crossref] [PubMed]
- Desai NR, Lee HJ. Diagnosis and management of malignant pleural effusions: state of the art in 2017. J Thorac Dis 2017;9:S1111-22. [Crossref] [PubMed]
- Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med 2016;74:330-5. [PubMed]
- Simoff MJ, Lally B, Slade MG, et al. Symptom management in patients with lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e455S-e497S.
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British thoracic society pleural disease guideline 2010. Thorax 2010;65 Suppl 2:ii32-40. [Crossref] [PubMed]
- Wallace MJ, Chin KW, Fletcher TB, et al. Quality improvement guidelines for percutaneous drainage/aspiration of abscess and fluid collections. J Vasc Interv Radiol 2010;21:431-5. [Crossref] [PubMed]
- Sunderland N, Maweni R, Akunuri S, et al. Re-expansion pulmonary oedema: a novel emergency therapeutic option. BMJ Case Rep 2016. [Crossref] [PubMed]
- Putnam JB Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86:1992-9. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Lee KA, Harvey JC, Reich H, et al. Management of malignant pleural effusions with pleuroperitoneal shunting. J Am Coll Surg 1994;178:586-8. [PubMed]
- Petrou M, Kaplan D, Goldstraw P. Management of recurrent malignant pleural effusions. The complementary role talc pleurodesis and pleuroperitoneal shunting. Cancer 1995;75:801-5. [Crossref] [PubMed]
- White MA, Agle SC, Padia RK, et al. Denver peritoneovenous shunts for the management of malignant ascites: a review of the literature in the post LeVeen Era. Am Surg 2011;77:1070-5. [PubMed]
- Tomiyama K, Takahashi M, Fujii T, et al. Improved quality of life for malignant ascites patients by Denver peritoneovenous shunts. Anticancer Res 2006;26:2393-5. [PubMed]
- Downing R, Black J, Windsor CW. Palliation of malignant ascites by the Denver peritoneovenous shunt. Ann R Coll Surg Engl 1984;66:340-3. [PubMed]
- Ghaffar MKA, Hassan MS, Mostafa MY. Value of implantable peritoneal ports in managing recurrent malignant ascites. The Egyptian Journal of Radiology and Nuclear Medicine 2014;45:417-22. [Crossref]
- Kriegel I, Daniel C, Falcou MC, et al. Use of a subcutaneous implantable pleural port in the management of recurrent malignant pleurisy: five-year experience based on 168 subcutaneous implantable pleural ports. J Palliat Med 2011;14:829-34. [Crossref] [PubMed]
- Yeh TS, Jan YY, Jeng LB, et al. Pyogenic liver abscesses in patients with malignant disease: a report of 52 cases treated at a single institution. Arch Surg 1998;133:242-5. [Crossref] [PubMed]
- Krokidis M, Hatzidakis A. Percutaneous minimally invasive treatment of malignant biliary strictures: current status. Cardiovasc Intervent Radiol 2014;37:316-23. [Crossref] [PubMed]
- Uberoi R, Das N, Moss J, et al. British society of interventional radiology: biliary drainage and stenting registry (BDSR). Cardiovasc Intervent Radiol 2012;35:127-38. [Crossref] [PubMed]
- Liberman D, McCormack M. Renal and urologic problems: management of ureteric obstruction. Curr Opin Support Palliat Care 2012;6:316-21. [Crossref] [PubMed]
- Dolan EA. Malignant bowel obstruction: a review of current treatment strategies. Am J Hosp Palliat Care 2011;28:576-82. [Crossref] [PubMed]
- Jiang TH, Sun XJ, Chen Y, et al. Percutaneous needle decompression in treatment of malignant small bowel obstruction. World J Gastroenterol 2015;21:2467-74. [Crossref] [PubMed]
- Kim YJ, Yoon CJ, Seong NJ, et al. Safety and efficacy of radiological percutaneous jejunostomy for decompression of malignant small bowel obstruction. Eur Radiol 2013;23:2747-53. [Crossref] [PubMed]
- Filippiadis DK, Tselikas L, Tsitskari M, et al. Percutaneous neurolysis for pain management in oncological patients. Cardiovasc Intervent Radiol 2019;42:791-9. [Crossref] [PubMed]
- Koyyalagunta D, Engle MP, Yu J, et al. The effectiveness of alcohol versus phenol based splanchnic nerve neurolysis for the treatment of intra-abdominal cancer pain. Pain Physician 2016;19:281-92. [PubMed]
- Kambadakone A, Thabet A, Gervais DA, et al. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics 2011;31:1599-621. [Crossref] [PubMed]
- Yarmohammadi H, Nakamoto DA, Azar N, et al. Percutaneous computed tomography guided cryoablation of the celiac plexus as an alternative treatment for intractable pain caused by pancreatic cancer. J Cancer Res Ther 2011;7:481-3. [Crossref] [PubMed]
- Wyse JM, Battat R, Sun S, et al. Practice guidelines for endoscopic ultrasound-guided celiac plexus neurolysis. Endosc Ultrasound 2017;6:369-75. [Crossref] [PubMed]
- Kelekis A, Cornelis FH, Tutton S, et al. Metastatic osseous pain control: bone ablation and cementoplasty. Semin Intervent Radiol 2017;34:328-36. [Crossref] [PubMed]
- Gangi A, Buy X. Percutaneous bone tumor management. Semin Intervent Radiol. 2010;27:124-36. [Crossref] [PubMed]
- Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain 1997;69:1-18. [Crossref] [PubMed]
- Deschamps F, Farouil G, Hakime A, et al. Cementoplasty of metastases of the proximal femur: is it a safe palliative option? J Vasc Interv Radiol 2012;23:1311-6. [Crossref] [PubMed]
- Anselmetti GC, Manca A, Ortega C, et al. Treatment of extraspinal painful bone metastases with percutaneous cementoplasty: a prospective study of 50 patients. Cardiovasc Intervent Radiol 2008;31:1165-73. [Crossref] [PubMed]
- Kelekis A, Lovblad KO, Mehdizade A, et al. Pelvic osteoplasty in osteolytic metastases: technical approach under fluoroscopic guidance and early clinical results. J Vasc Interv Radiol 2005;16:81-8. [Crossref] [PubMed]
- Kelekis A, Filippiadis D, Anselmetti G, et al. Percutaneous augmented peripheral osteoplasty in long bones of oncologic patients for pain reduction and prevention of impeding pathologic fracture: the rebar concept. Cardiovasc Intervent Radiol 2016;39:90-6. [Crossref] [PubMed]