
PD-L1 expression and efficacy of pembrolizumab as monotherapy in NSCLC
Introduction
The introduction of programmed death-1 (PD-1) and the programmed death-ligand 1 (PD-L1) inhibitors have significantly impacted cancer therapy over a relatively short period of time. Within the past 5 years, the PD-1/PD-L1 inhibitors have become standard of care for 16 different cancer and tumor-agnostic indications. Some of the largest clinical development programmers ever seen in oncology have supported this expansion. Not only has the number of clinical indications been expanded, but also the number of compounds. By the end of 2019, nine different PD-1/PD-L1 inhibitors have obtained regulatory approval in different countries worldwide; however, three of these compounds are currently available in China only (1). The first immune checkpoint inhibitors to be approved by the regulatory authorities were pembrolizumab (Keytruda, Merck Sharp & Dohme) and nivolumab (Opdivo, Bristol-Myers Squibb). In 2014, both compounds obtained approval for treatment of patients with advanced melanoma, which in 2015, was followed by an approval for second-line treatment of metastatic non-small cell lung cancer (NSCLC) (2).
For pembrolizumab, the first NSCLC indication was based on data from the KEYNOTE-001 study, which was an expanded large-scale phase Ib study. Besides assessing the safety and efficacy of pembrolizumab, this study also aimed at clinical validating the immunohistochemical (IHC) 22C3 assay for the determination of PD-L1 tumor expression (3). The KEYNOTE-001 study showed that the response to pembrolizumab was positively related to the PD-L1 expression level and based on the outcome data from the study, a tumor proportion score (TPS) of 50% was selected as the assay cut-off value. In parallel to the approval of pembrolizumab for second-line treatment of metastatic NSCLC, the PD-L1 22C3 IHC pharmDx assay (Dako) was granted approval as the companion diagnostic (2). In the subsequent KEYNOTE-010 study, which was a phase II/III study in second-line metastatic NSCLC, the TPS cut-off value was lowered to 1% (4). However, when pembrolizumab in the KEYNOTE-024 study moved into the first-line setting, the 50% PD-L1 TPS was reintroduced as cut-off value using the PD-L1 22C3 IHC pharmDx assay.
KEYNOTE-024
In the KEYNOTE-024 phase III study, 305 metastatic NSCLC patients, without EFGR or ALK tumor aberrations, and with a PD-L1 TPS ≥50% were randomized to receive pembrolizumab or platinum-based chemotherapy using an open-label design (5-7). The assessment of the PD-L1 tumor expression was performed by the PD-L1 22C3 IHC pharmDx assay. Patients assigned to chemotherapy were allowed to cross over to pembrolizumab, which was the situation for 82 patients. For the primary study endpoint of progression free survival (PFS), pembrolizumab showed superiority over platinum-based chemotherapy. The median PFS was 10.3 months in the group of patients who received pembrolizumab and 6.0 months in the chemotherapy group [hazard ratio (HR) 0.50 (95% CI: 0.37–0.68)]. Overall survival (OS) was a secondary endpoint and based on a median follow-up time of 25.2 months, the median OS for the pembrolizumab group was 30.0 months and for the chemotherapy group 14.2 months [HR 0.63; 95% CI: 0.47–0.86]. When the data was adjusted for crossover, the HR) for OS for pembrolizumab versus chemotherapy was [HR 0.49 (95% CI: 0.34–0.69)]. A likewise positive result was seen with respect to the objective response rate (ORR). Here the pembrolizumab group showed an ORR of 44.8% compared to 27.8% for the patients who received platinum-based chemotherapy. Based on the results from this study, it was concluded that first-line pembrolizumab monotherapy in patients with metastatic NSCLC and a PD-L1 TPS ≥50% showed a PFS and OS benefit over platinum-based chemotherapy (5-8). Furthermore, pembrolizumab monotherapy was associated with fewer treatment related grade 3 to 5 adverse events compared to chemotherapy (6). Based on the results from the KEYNOTE-024 study, testing for PD-L1 expression is now routinely performed in patients with newly diagnosed NSCLC, and in many countries pembrolizumab monotherapy has become standard treatment for patients with a PD-L1 TPS ≥50% (8).
KEYNOTE-042
In the KEYNOTE-042 study, pembrolizumab was further evaluated as monotherapy in an open labeled randomized phase III multicentre study conducted in more than 30 countries worldwide, including China (9). In the study, 1275 NSCLC patients, without EGFR or ALK tumor aberrations, were randomized to receive pembrolizumab (N=638) or platinum-based chemotherapy (N=637). The assessment of the PD-L1 expression was performed by the PD-L1 22C3 IHC pharmDx assay. The design of the KEYNOTE-042 study was similar to KEYNOTE-024, but in contrast to this, it did not permit crossover to pembrolizumab for patients in the chemotherapy arm. However, the most important difference between the two studies was that KEYNOTE-042 allowed enrollment of patients with a PD-L1 TPS ≥1%. The primary study endpoint was OS with PFS as a secondary endpoint. Furthermore, the study was designed to evaluate the study endpoints in relation to three different PD-L1 TPS cohorts defined as; TPS ≥50%, TPS ≥20% and TPS ≥1%. In addition to these three expression levels, a fourth PD-L1 TPS of 1–49% was included as an exploratory endpoint.
For OS, all three prespecified PD-L1 TPS cohorts showed that treatment with pembrolizumab was superior to platinum-based chemotherapy. The HR’s for the three PD-L1 TPS were in favor of pembrolizumab, as shown in Table 1, and similar for the corresponding median OS, as shown in Figure 1. When the data for the three cut-off levels are compared there seems to be a positive relation between PD-L1 expression and the efficacy of pembrolizumab. The median OS increases with increasing PD-L1 expression while the HR’s decreases. As shown in Figure 1, the median OS for PD-L1 TPS ≥1% was 16.7 months which increased to 20.0 months for PD-L1 TPS ≥50% (9). However, it is important to note that the increased survival benefit across the different PD-L1 expression cohorts was mainly driven by the PD-L1 TPS ≥50% group (8). The analyses of data from the PD-L1 TPS ≥1% and TPS ≥20% cohorts are confounded by the data from the PD-L1 TPS ≥50% cohort. It becomes particularly clear when looking at the exploratory analysis of the PD-L1 TPS 1-49% data. The median OS for this cohort was 13.4 months, which is similar to platinum-based chemotherapy and the HR for the comparison was insignificant [HR 0.92 (95% CI, 0.77–1.11)]. If the KEYNOTE-042 study should have been able to explore a potential differential response to pembrolizumab monotherapy, it should have been analyzed differently. The data should have been divided into non-overlapping intervals as the following: PD-L1 TPS 1–19%, PD-L1 TPS 20–49%, and PD-L1 TPS ≥50%. However; whether this would have provided additional information is doubtful, as the comparison of PD-L1 TPS 1–49% and PD-L1 TPS ≥50% clearly indicates that pembrolizumab monotherapy is superior in patients with a PD-L1 TPS ≥50%. Anyhow, it would have been interesting to see if there was a difference in the efficacy of pembrolizumab given as monotherapy to patients with a PD-L1 TPS 1–19% or PD-L1 TPS 20–49%, respectively. Based on the size and the design of the KEYNOTE-042 study, it should have been able to give us this answer.
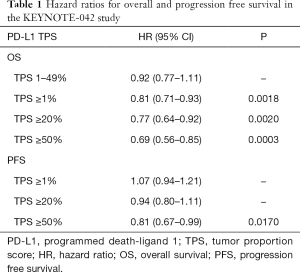
Full table
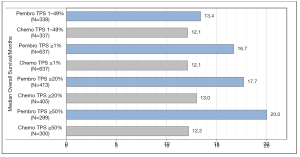
With regard to the secondary endpoint of PFS, the results from the pembrolizumab and chemotherapy arms were similar, as it appears from the HRs in Table 1. In the PD-L1 TPS ≥50% cohort, the median PFS was 7.1 months in the pembrolizumab group and 6.4 months in the chemotherapy group, which was the largest difference observed between the two treatment arms for any of the TPS cohorts (9). As in the KEYNOTE-024 study, the safety data was in favor of pembrolizumab monotherapy. There were fewer observed treatment related adverse events of grade 3 or more in the pembrolizumab group compared to the platinum-based chemotherapy group. Overall, the KEYNOTE-042 study should be seen as a study confirming the results of KEYNOTE-024, and the conclusion must still be that a PD-L1 TPS ≥50% cut-off value is the most optimal patient selection criterion for pembrolizumab monotherapy as first-line treatment of metastatic NSCLC (8).
PD-L1 IHC 22C3 pharmDx
The PD-L1 IHC 22C3 pharmDx assay is a qualitative IHC assay, using the monoclonal mouse anti-PD-L1 clone 22C3 (10,11). In 2015 when the US Food and Drug Administration (FDA) approved pembrolizumab for second-line treatment of patients with metastatic NSCLC, they simultaneously approved the PD-L1 22C3 IHC pharmDx assay as the companion diagnostic. The assay is intended for detection of the PD-L1 protein in formalin-fixed, paraffin-embedded NSCLC tissue specimens. The level of PD-L1 expression is expressed as a TPS, which is defined as viable tumor cells showing partial or complete membrane staining relative to all viable tumor cells present in the sample. A specimen having a TPS ≥50% was originally considered PD-L1 positive but later on the cut-off level was lowered to a TPS ≥1%, likely based on the results from the KEYNOTE-010 study (4). In the US FDA Summary of Safety and Effectiveness Data document for the PD-L1 IHC 22C3 pharmDx assay, it is further stated that the assay is indicated as an aid in identifying NSCLC patients for treatment with pembrolizumab (10).
The PD-L1 IHC 22C3 pharmDx assay stand out as one of the very few companion diagnostic assays where a proper cut-off selection has been performed based on clinical outcome data and receiver operating characteristic (ROC) analysis (11-13). The selection of the clinical cut-off value for the assay was based on data from the phase 1b KEYNOTE-001 study (3). This study aimed to evaluate the safety and efficacy of pembrolizumab and to clinically validate the PD-L1 IHC 22C3 assay. In relation to this validation, the 495 NSCLC patients enrolled in the study were divided into two groups; one third of the patients was assigned to a training group and two thirds to a validation group. The data from the training group was used to define the clinical cut-off value for the PD-L1 IHC 22C3 pharmDx assay, and based on ROC analysis, a PD-L1 TPS ≥50% was selected as the most optimal assay cut-off value. Subsequently, when the efficacy data from the validation group was analyzed, using the selected cut-off value, the results showed that patients with a TPS ≥50% had longer median PFS and OS as well as a higher ORR compared to patients with a TPS <50% (2). With a TPS ≥50% as cut-off value, the area under the ROC curve was 0.743, which corresponds to a clinical sensitivity of 70.4% and a clinical specificity of 79.0% (3,7,12). As described above, the clinical utility of the PD-L1 TPS ≥50% cut-off value has been documented in both the first-line and second-line setting (4,6,9). Furthermore, the recent published five-year data form the KEYNOTE-001 study has likewise confirmed the clinical utility of the selected PD-L1 cut-off value (14). For the treatment-naïve patients with a PD-L1 TPS ≥50%, the median OS was 35.4 months compared to 19.5 months in the group of patients with a PD-L1 TPS 1–49%. For the previously treated patients with a PD-L1 TPS ≥50%, the median OS was 15.4 months compared to 8.5 months for patients with a PD-L1 TPS 1–49%. Based on the results from the different first- and second-line studies with pembrolizumab monotherapy in NSCLC, a PD-L1 TPS ≥1% has subsequently been approved by the US FDA as a general cut-off value (15). As for the original selection of the PD-L1 TPS ≥50% cut-off value, it would be desirable to have similar data on the clinical sensitivity and specificity for the PD-L1 TPS ≥1%.
Pembrolizumab as monotherapy in NSCLC
Looking at the way that pembrolizumab monotherapy for NSCLC has been developed, the PD-L1 IHC 22C3 pharmDx assay has played a key role. For both the first- and second-line indications, the initial step was to demonstrate safety and efficacy using PD-L1 TPS ≥50% as cut-off value and then subsequently widen the indications to include patients with PD-L1 TPS ≥1%. Looking at the different clinical studies conducted with pembrolizumab monotherapy, they seem to confirm a differential response depending on the tumor PD-L1 expression level (3-6,9). Across these studies, a greater survival benefit has been shown for patients with a PD-L1 TPS ≥50% compared to a PD-L1 TPS <50%. This survival benefit was observed in both the first and second-line setting. Based on the data from the KEYNOTE-024 and the KEYNOTE-042 studies, it is recommended that PD-L1 TPS ≥50% should be used as selection criteria for first-line monotherapy with pembrolizumab in metastatic NSCLC (8). For patients with a lower PD-L1 tumor expression, a combination with chemotherapy is an option either with pembrolizumab or one of the other PD-1/PD-L1 inhibitors approved for this indication.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco.2020.01.03). Dr. Jørgensen reports personal fees from null, personal fees from null, personal fees from null, personal fees from null, personal fees from null, personal fees from null, personal fees from null, personal fees from null, during the conduct of the study; personal fees from null, personal fees from null, personal fees from null, personal fees from null, personal fees from null, outside the submitted work.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bonora M, Wieckowsk MR, Chinopoulos C, et al. Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene 2015;34:1608. [Crossref] [PubMed]
- Jørgensen JT. Companion diagnostic assays for PD-1/PD-L1 checkpoint inhibitors in NSCLC. Expert Rev Mol Diagn 2016;16:131-3. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Jørgensen JT, Nielsen KB. Companion and complementary diagnostics for first-line immune checkpoint inhibitor treatment in non-small cell lung cancer. Transl Lung Cancer Res 2018;7:S95-9. [Crossref] [PubMed]
- Peters S, Reck M, Smit EF, et al. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann Oncol 2019;30:884-96. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- U.S. Food and Drug Administration. Summary of Safety and Effectiveness Data (SSED) for PD-L1 IHC 22C3 pharmDx. October 2, 2015. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf15/p150013b.pdf. Accessed November 18, 2019.
- Hersom M, Jørgensen JT. Companion and Complementary Diagnostics-Focus on PD-L1 Expression Assays for PD-1/PD-L1 Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Ther Drug Monit 2018;40:9-16. [PubMed]
- Dolled-Filhart M, Roach C, Toland G, et al. Development of a Companion Diagnostic for Pembrolizumab in Non-Small Cell Lung Cancer Using Immunohistochemistry for Programmed Death Ligand-1. Arch Pathol Lab Med 2016;140:1243-9. [Crossref] [PubMed]
- Roach C, Zhang N, Corigliano E, et al. Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Non-Small-cell Lung Cancer. Appl Immunohistochem Mol Morphol 2016;24:392-7. [Crossref] [PubMed]
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518-27. [Crossref] [PubMed]
- U.S. Food and Drug Administration. Summary of Safety and Effectiveness Data (SSED) for PD-L1 IHC 22C3 pharmDx. July 30, 2019. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150013S016B.pdf. Accessed November 20, 2019.


